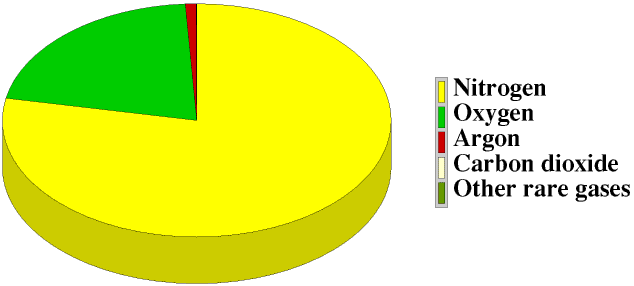
Dry unpolluted air
is usually considered to contain about

The atmosphere also naturally contains dusts and pollens and a large but variable amount of water vapour. Today it also contains significant amounts of a large number of pollutants. This is further discussed in the page on Atmospheric Pollution.
Nitrogen is widely used to replace air where there might be a risk of explosion, for example when a supertanker is unloading, as the petroleum is taken out of the tanks they are filled with nitrogen. It is also used in food storage units to prevent food going bad. Liquid nitrogen (196oC ) is widely used to keep things very cold. Liquid nitrogen is very cheap because it is a by-product of the production of liquid oxygen - see below.
Oxygen is used in respiration and combustion, and also has a very large number of uses in industry: steel-making, flame-welding etc.
Argon is used in light bulbs and to produce an inert atmosphere for welding etc.
Carbon dioxide is used in fire extinguishers and fizzy drinks and for many other purposes.
Helium is much lighter than air and is used in airships because although it is very much more expensive than hydrogen it is non-flammable. Deep sea divers also often breathe a mixture of oxygen and helium to avoid decompression sickness (“the bends”). Such a mixture makes your voice go squeaky. A mixture of helium and air or oxygen is harmless but if you breathed pure helium, for example from a balloon, for more than one short puff you would harm yourself because you need oxygen to live. Liquid helium (-269oC or 4 K) is used in very low temperature experiments. Hydrogen and helium are light enough to escape into space, so hydrogen does not exist in the Earth's atmosphere; helium exists there only because it is being continuously produced by the radioactive decay of all alpha-emitters see the Page on radioactivity.
Krypton is used in some high-powered lights.
Neon is used in red lights such as shop displays and advertisements.
Radon is radio-active and is used as a tracer, for example to detect leaks in underground gas pipelines. It is produced by the decay of radio-active substances present in rocks such as granite, and in certain parts of England such as Cornwall, where there is a lot of granite near the surface, cellars and underfloor spaces in houses need to be well-ventilated to avoid dangerous levels of radio-active gases building up.
Xenon is used in stroboscopes and other special types of lighting.
All the gases present in the atmosphere, except helium, carbon dioxide and radon, are manufactured by separating them from air. Carbon dioxide is obtained as a by-product of brewing and by other industrial processes, while radon is collected from the radioactive decay products of certain substances. Helium occurs naturally in rocks in certain parts of the United States.
For a page on the storage of these, and other, gases please click hereIf we cool carbon dioxide at atmospheric pressure it turns into not a liquid but a solid, as explained on the Page on storage of gases.. All the other gases in the atmosphere turn into liquids if cooled enough. Their boiling points (in oC at atmospheric pressure) are about
| Radon | -62 |
| Xenon | -108 |
| Krypton | -154 |
| Oxygen | -182 |
| Argon | -186 |
| Nitrogen | -196 |
| Neon | -246 |
| Helium | -269 |
These gases (except radon and helium) are manufactured by cooling the air until it liquefies and then separating the gases in the liquid air by fractional distillation. Liquid nitrogen is not expensive because industry needs very large quantities of liquid (or gaseous) oxygen, but for every tonne of liquid oxygen produced four tonnes of liquid nitrogen are produced.
First the air is filtered to remove dust and pollen, and treated to remove water vapour and carbon dioxide (which would solidify and block the pipes when the air was cooled). Then the air is compressed to a very high pressure. This makes it very hot and it is cooled with water back to normal temperature. Then the pressure is released and as the air expands it cools to about -200oC. At this temperature the nitrogen, oxygen, argon, krypton, radon and xenon liquefy. This mixture is called liquid air, and we can separate the gases in it by fractional distillation. To make neon we take the gas that remains after all the other gases have liquified and compress it to a very high pressure. We then cool it, first with water and then with liquid nitrogen, and then release the pressure to cool and liquefy it and then fractionally distil it as before.
Helium occurs naturally in very large quantities in rocks in certain parts of the USA and other countries. It occurs in the atmosphere in such minute quantities that it is quite uneconomic to produce it by separating it from the gas left behind after liquid air has been produced.
© Barry Gray April 2011