Bubbles are great fun, and each August Gillingham United Reformed Church has a Bubbles Afternoon. For lots more about Fun with Bubbles you can visit Doctor Zigs wonderful web site.
This Page provides a simple introduction to the science behind bubbles and soap films, and the instructions for floating a needle on water (something best done at home) which is the key to understanding how bubbles work.
If we walk over a sandy beach with bare feet after we have been swimming in the sea some grains of sand may stick to our feet: these grains of sand are not very big! But atoms and molecules are unimaginably tiny: if we cover a football (soccer) pitch with a layer of sand deep enough to cover the goal posts, there are more molecules of silicon dioxide in one grain of sand than there are grains of sand on the football pitch!
Atoms and molecules attract each other, the closer they are the bigger the attraction. In solids and liquids they are very close to each other but in air and other gases they are much further apart.Here is a not quite full beaker of water (showing the atoms and molecules but not to scale!).
In the middle of the beaker the molecules of water are attracted in every direction, but at the surface, where the water meets the air, they are being attracted sideways and downwards but not upwards, so they behave differently: it is as though the molecules at the surface of the water are part of a very tightly stretched skin, a bit like a balloon. This effect is called surface tension. Surface tension exists in all liquids.
Surface tension is always trying to pull the liquid into the shape with the smallest possible surface area - for a droplet of water, or any other liquid, this is usually a sphere. In the Old Days lead shot for muskets and other guns was made in a Shot Tower. This was a tall hollow tower. Molten lead was poured from a cauldron at the top, and as the liquid lead fell down inside the tower it broke up into spherical droplets which solidified before they reached the bottom. At the bottom the pellets fell into a tub of water. They were all spherical but not all the same size, so they were then passed through different sized sieves to sort them into the right sizes for the different types of gun. The rest of this Page is only about surface tension in water.
Where the water comes into contact with a solid, for example the sides of a glass or a straw, the water molecules and solid molecules are very strongly attracted to each other so different things happen. The narrower the straw the further the water climbs up inside it. This is not discussed further here but it is very important to you if you happen to be the wick of a candle or a tree.
Try to notice this next time you have a drink with a straw.
For this experiment you will need an adult (adults do not like children playing with pointy things but the real reason you need one is to show how clever you are), a bowl of clean tap water, a sewing needle and a paper tissue or a piece of kitchen roll. (And a drop of washing up liquid, but only at the very end.)
Very carefully, without getting your fingers wet, put the piece of paper on the surface of the water. It will stay on the surface but start to soak up water. Now, again without getting your fingers wet, put the needle onto the paper. After a few more seconds the paper will have soaked up so much water that it will sink, but the needle does not break the surface film so floats on it! (If the paper takes more than about twenty seconds to sink you can push one edge of the paper down with something like the handle of a clean metal spoon but not your fingers.) If you look very carefully you can see the dent the needle makes in the water, like pushing your finger into a balloon. If you have magnetised the needle first you can use it as a simple compass - how to do this is described on another Page.
Some small insects and other small animals can walk on water in this way - a web search on pond skaters will produce lots of lovely pictures.
Many animals including fishes and dragonfly larvae and tadpoles live in water, and these get the oxygen they need to live from the oxygen dissolved in the water. If the water loses its oxygen (becomes eutrophied) they will die - how this can happen is described here. (Whales and dolphins are mammals, so although they live in water they have to come to the surface to breathe air.) Mosquito larvae also live in water but they breathe air through a narrow tube in their tails by using the surface tension of the water to hang from them! So they can live in water too polluted for anything else to live, particularly animals such as fishes which might eat them!
You can see some pictures by doing a web search on mosquito larvae.
Once you have watched the needle floating for long enough and shown all your family how clever you are you can end the experiment in a really spectacular way which explains soap bubbles!
Put one drop of undiluted washing up liquid onto a small plate, then touch this drop with something like the handle of a metal teaspoon, then just touch this part of the teaspoon to the surface of the water in the bowl, well away from where the needle is floating. The needle will sink instantly!
Water with soap (or other detergent) in it has a much lower surface tension than clean water, and this affects the way it behaves when it comes into contact with something like our skin or our hair or our clothes or our dirty dishes, which is of course why we use soap for washing our skin, our hair, our clothes and our dirty dishes. It is the much lower surface tension of soap solution which makes soap films and soap bubbles possible.
Soap bubbles can float away on the wind, and if they come into contact with a tent they will have the same effect on the tent fabric that they have your clothes, that is, make it less waterproof, so you should not make bubbles on a camping site.
Ducks (real and plastic), and you, and aircraft carriers, are much too heavy not to break the surface tension film: we can float in water because of our buoyancy not because of surface tension.
Before you do anything else take the needle out of the bowl and dry it and put it away safely; if you are going to do the experiment again at another time put a piece of BluTack® or similar on the point to make it safe.
You can make a ”bubble mixture” for making bubbles and soap films in many ways, and the cheapest and easiest is by mixing ordinary washing up liquid with tap water, but if you want really big bubbles and soap films and want them to last a long time it is better to use a proper bubble mixture. You can buy bubble toys, and these usually come with a small supply of ready-mixed solution, and you can also buy refills in various sizes. For the very best results you should buy a special concentrated liquid and mix it with rain water or, in school, distilled or deionised water.
Always remove any froth or foam from the surface of the mixture before using it, a paper tissue is useful for this. Foams and froths are described later.
Mixing soap with water lowers the surface tension of the water so the water molecules are less strongly attreaced to each other. But it does not change the way they are attracted to something solid, for example a metal wire.
If we put a metal ring into soapy water when we pull it out we shall have a soap film in it.
The frame does not have to be round, or even flat: try bending a metal wire clothes hanger into a really twisty shape and then putting it into the bubble mixture. The soap film will always take the shape, often a very elaborate 3-D shape, with the smallest possible surface area. Rumour has it that the spectacular roof of the Opera House in Sydney, Australia was designed in this way!
If you want to study the wonderful colours you can see in a soap film this is the best way to make it, because you can hold it at just the right angle and close to your eye.
A soap film cannot have an edge, if it separates from its frame its surface tension will pull it into a tiny round droplet - it will burst. It must end on a solid or join with another soap film, or of course become a bubble. Three, but never more than three, soap films can meet, and the angle between them where they meet is always 120°, but the films do not have to be flat.
You can have an arrangement of lots of soap films but only three can join along the same line. Your School might have a soap film kit allowing you to do experiments with soap films for yourself.
A soap film is fixed to its frame, but a bubble follows the air currents. If it hits another bubble or a soap film it will usually join with it.
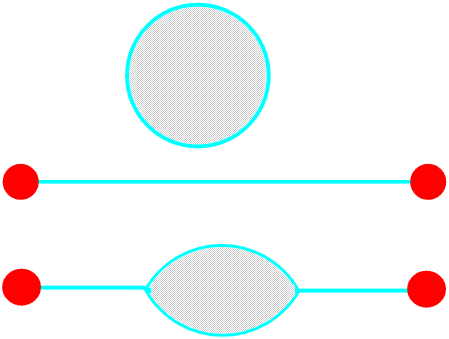
Remember that the angles between soap films are always 120°
If a bubble hits a solid object it may just stick to it, or it may burst, or, and most people find this surprising, it may bounce off: you can buy special bats like table tennis bats to play games with bubbles!
A soap film or bubble will burst if it is damaged, just like a balloon. But they are quite stretchy so they can change their shape without bursting, again just like a balloon.
If you blow on a soap film you will change its shape and it may then either break away from its frame and become a bubble or burst. But most soap films and bubbles come to an end because the water in them is evaporating.
If you have several soap films and bubbles joined together they will not all burst at the same time: as each one bursts the surface tension in the others pulls them into different shapes. Bubbles usually last longer than soap films because in a soap film the water is evaporating from both sides whereas for a bubble the water is evaporating only from the outside. So if you start with a “square” bubble inside a cube you will end up with a “spherical” one!
If we stir or shake soapy water we may get lots of very tiny bubbles all joined together - this is called a foam or a froth. This takes a long time to disappear because the inside bubbles are protected from the air by the outside ones and so do not evaporate so quickly.
We can also get froth on, for example, beer or milk - both do contain water of course. Many adults do not like foam on their beer, but many children like frothy milk.
If you just want to have fun with bubbles foam and froth are a nuisance, but they do have their uses, for example in putting out or preventing fires involving burning liquids after an aircraft has crashed and damaged its fuel tanks.
When light falls on something some of the light is reflected off it, some is absorbed by it and some passes through it. Most of the light falling on a bubble or soap film passes through it - it is transparent - but just enough is reflected off it to allow us to see it, and just enough light is absorbed by it for it to leave a shadow on the ground on a sunny day.

The reason why we get colours in bubbles and soap films is slightly more technical and is given on another Page (under construction). But some of the colours in the above picture of the boy are there because he is eating an ice lolly!

Barry Gray last revised July 2022