
If you take off a nylon shirt in a quiet dark room you can both hear and see the sparks flying. I am told you get the same effect with a nylon blouse but I have never tried this for myself. This effect is due to “static electricity”.
Two thousand five hundred years ago the Ancient Greeks did not have nylon, so they had to make their clothes out of natural materials such as wool, cotton, linen, silk, leather and fur. Jewellery was made mainly from gold and silver, with precious and semi-precious stones such as rubies, emeralds, sapphires, opals, amethysts, beryls and jade. This sort of jewellery does not usually produce static electricity. But Greek ladies were also very fond of amber jewellery, and if a lady walked down the road wearing a dress with a fur collar and an amber necklace the sparks really flew!
The Greek for amber is elektron, hence our word electricity.
It is “obvious” that if you rub amber with fur something happens to the amber, it is much less obvious that something also happens to the fur. Similarly if you rub glass with silk it is “obvious” that something happens to the glass but much less obvious that something also happens to the silk. So for the next two thousand years electricity (what today we would call static electricity) was about glass and amber not silk and fur. Even today you can rub a balloon against your jumper and stick it to the wall or pick up bits of paper with it and everyone thinks you are clever, but no one thinks about your jumper.
By about 1730 it was thought that there were two different sorts of electricity, one on materials such as glass and the other on materials such as amber, and that these different types repelled each other. In 1733 the French scientist Charles du Fay (1698 - 1739) named these two types of static electricity “vitreous” (from the Latin for glass) and “resinous” (amber being from a resin), but later they were renamed “positive” and “negative”. They could have decided to say that resinous was positive and vitreous was negative but no, they had to decide that vitreous was positive and resinous was negative, and today this causes endless confusion to young people learning about electrolysis and semi-conductors.
Today when we talk about electricity we usually mean the sort of electricity which flows along wires, from positive to negative. But until the invention of the battery (in 1800) and the dynamo (in 1831) the only sort of electricity people knew about was static electricity. It was the English scientist Michael Faraday (1791 - 1867) who was able to show that vitreous and resinous electricity, lightning, the electricity produced by a battery, and the electricity produced by a dynamo, are all the same.
The only problem is that once we decided to say that glass rubbed with silk has a positive charge then the electron, discovered in 1887, has a negative charge, so a current passes from positive to negative only because the electrons which are actually carrying it are passing from negative to positive!
Well, that is the traditional, Euro-centric, view of the history of electricity. Actually it is so easy to make a simple battery (today you can buy a kit to make a digital clock powered by two metal rods stuck into a piece of potato. Potato not included) that more and more scientists and historians are now thinking that it is quite possible that many ancient people were making them, even using them to silver-plate copper jewellery!

Lots of solids have an ionic structure. The solid is made up of a lattice of positively and negatively charged ions held in place by the electrostatic attraction between them. In the solid these ions are not free to move away from their place in the lattice.
If however we melt the material or dissolve it in a certain type of solvent (a polar solvent, almost always water) then the ions are free to move, and if we then put two rods, one with a positive charge and one with a negative charge, into the liquid the positively charged ions will move towards the negatively charged rod and the negatively charged ions will move towards the positively charged rod. The charged rods are called electrodes, the positively charged rod is the anode (from the Greek for “way down”) and the negatively charged rod is the cathode (from the Greek for “way up”.) The liquid is called the electrolyte. Electrolytes are mainly molten metallic salts, oxides and hydroxides, and aqueous solutions of acids, alkalis and metallic salts. The passing of an electric current through an electrolyte is called electrolysis. Electrolysis involves two separate chemical changes, one at the cathode and one at the anode. These are called the half reactions.
The ions that move towards the anode are called anions and those that move towards the cathode are called cations - pronounced cat ions, as if you could not work it out!
What the electrodes are made of may affect what happens at them. Sometimes we want the material from which the electrodes are made to take part in the chemical changes, but if we do not we usually use carbon (graphite) electrodes. If the substances being used or produced are very corrosive we sometimes use platinum electrodes.

When we melt lead bromide it becomes lead ions and bromine ions.
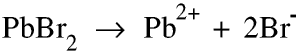
This is what happens when we use carbon electrodes.
The bromine ions move towards the anode. Here they each give up an electron to the anode.
The lead ions move towards the cathode. Here they gain 2 electrons.
Electrons are thus gained by the anode and lost by the cathode so there is a flow of electrons from the cathode to the anode and a current from the anode (+) to the cathode (-).
At the temperature of the liquid lead bromide the bromine produced will be a gas (vapour) and the lead will be a liquid.
Bromine is produced at the anode and lead at the cathode. But we must always explain what is happening at the anode and cathode separately.

The sodium hydroxide separates into sodium (Na+) and hydroxide (OH-) ions.
This is what happens if we use carbon electrodes.
The sodium ions move towards the cathode. Here they gain an electron
The overall reaction is therefore
This was the way the English scientist Sir Humphrey Davy (1778 - 1829) first made sodium, and also potassium, in 1807.
Many other substances can be melted and electrolysed in this way, but not all carbonates, sulphates and nitrates (and other similar salts containing oxygen) can be electrolysed in this way because many decompose rather than melt when they are heated, for example calcium carbonate decomposes when heated to produce calcium oxide and carbon dioxide - this is discussed on the Lime Cycle Page. However if we electrolyse, say, molten sodium sulphate we get sodium at the cathode, as for sodium hydroxide. But “sulphate” cannot exist except as sulphate ions, so at the anode we get sulphur dioxide and oxygen.

Electrolysis of aqueous solutions is not quite so simple. If for example we dissolve copper sulphate in water the solution will contain not only copper ions and sulphate ions but also hydrogen ions and hydroxide ions from the water - this is discussed in greater detail, for Advanced Readers only, at the end of this Page. What happens may also depend upon the concentration of the solution and what the electrodes are made of.
If we use carbon electrodes this is what happens.The copper and hydrogen ions move towards the cathode. Hydrogen is higher than copper in the order of reactivity so it is the copper ions rather than the hydrogen ions which gain electrons. This is discussed later on this Page.
The carbon cathode becomes coated with copper.
The sulphate ions and the hydroxide ions move towards the anode. For reasons explained below on this Page, here it is the hydroxide ions which give up their electrons, forming oxygen and water.
If however we use copper electrodes this is what happens.
The copper cathode becomes coated with copper, as before, and, let’s face it, a piece of copper covered with copper is rather more useful than a piece of carbon covered with copper.
The sulphate and hydroxide ions move towards the anode. Copper is less electro-positive than these, so instead of the hydroxide ions losing electrons the copper on the anode does, forming copper ions which move into the solution.
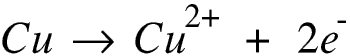
In effect the copper anode dissolves, so copper is transferred from the anode onto the cathode, but no new substances are produced.
This process is used for making the very pure copper needed for the copper wires in electronic equipment. The anode is a lump of impure copper and the cathode a small piece of very pure copper. Copper dissolves from the anode and is deposited on the cathode, while the impurities sink to the bottom of the tank as an anode slime. This slime contains the other metals which were present in the copper ore, which usually include silver, gold and platinum!
We can also use the same process to plate a metal with another (less reactive) metal. We can silver-plate a nickel-steel teapot by using the teapot as the cathode, a piece of pure silver as the anode and silver nitrate solution as the electrolyte. The teapot is usually stamped EPNS for electroplated nickel steel. When it is new a silver-plated teapot looks exactly the same as one made out of solid silver but it is not as heavy, and the plating will eventually wear away to show the base metal underneath.
We can also nickel- and chromium-plate objects in the same way.
The ancient people did not of course have nickel-steel, but you can also silver-plate a copper object, and there is more and more evidence every year to suggest that the ancient people were doing just that.

If we have a dilute solution of sodium chloride and use carbon electrodes the sodium and hydrogen ions move towards the cathode. Sodium is more reactive that hydrogen so it is the hydrogen ions which gain electrons.
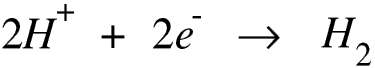
The chloride and hydroxide ions move towards the anode. Here the hydroxide ions lose their electrons forming water and oxygen.
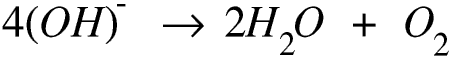
The overall reaction is therefore
The solution just gets more concentrated.
If we carry out this reaction on anything but a very small scale we must make sure the building is very well ventilated, otherwise we shall get an explosive mixture of hydrogen and oxygen building up. This mixture is known as electrolytic gas; it is often produced when we electrolyse acids and dilute solutions of salts, and also when we recharge old-style lead-acid car batteries.
If we use concentrated sodium chloride and carbon electrodes we still get hydrogen at the cathode, but at the anode some of the chloride ions may lose an electron.
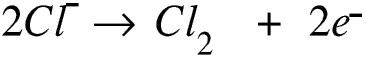
Initially there are equal numbers of sodium ions and chloride ions, and of course also hydrogen and hydroxide ions from the water. If we now remove chloride ions we are left with hydrogen ions, sodium ions and hydroxide ions, that is, sodium hydroxide and water.
The overall reaction is therefore
Sodium hypochlorite is a useful product in its own right, and is used in bleaches etc, but more usually we want sodium hydroxide not contaminated with chlorine. There are several different ways of keeping the sodium hydroxide and chlorine apart: one is, or at any rate was, to use mercury as the cathode. What follows is included here only because it is mentioned in some school textbooks, but usually not explained very well.
A mixture of two or more metals, or a metal and another substance, is called an alloy. Bronze is an alloy of copper and tin. Alloys often have physical properties very different from the metals in them, which is of course why they are so useful. This is discussed on the Page on Metals and Alloys.
Mercury is the only metal which is a liquid at room temperature. It forms an alloy immediately it comes into contact with any other metal. This is called amalgamating, and an alloy of mercury and another metal is called an amalgam. The effect is instantaneous, and most metals, but particularly gold, are ruined by coming into contact with mercury.
If we are electrolysing concentrated sodium chloride solution using a carbon cathode, sodium cannot be formed at the cathode because it would immediately react with the water to form sodium hydroxide and hydrogen. If however we use a mercury cathode sodium can be formed because it will amalgamate with the mercury rather than react with the water, so we shall get sodium at the cathode, in the form of a sodium amalgam.
The overall reaction is therefore
This process could be used to produce sodium, or many other metals, although it is not; its real use is, or was, to produce chlorine-free sodium hydroxide. The mercury forming the cathode is at the bottom of the tank and the sodium chloride solution is floating on it. The mercury is continuously circulated through the tank. Outside the tank it is mixed thoroughly with water. The sodium reacts with the water to form sodium hydroxide and the mercury is then recirculated back into the tank.
This process was once widely used, but is used much less today because of concerns over the safety of mercury.

Electrolysis is used in the extraction of many metals, including sodium, potassium, magnesium and aluminium, in the purification of copper, and in electroplating, and in the manufacture of many materials including sodium hydroxide, sodium hypochlorite and chlorine.

We measure the quantity of water in litres, and the rate of flow of water through a pipe in litres per minute. In the same way, we measure the quantity of electricity in coulombs (C) and the rate of flow of electricity through a wire in coulombs per second. We call an electric current of one coulomb per second one ampere (A).
If we electrolyse a solution by passing a current of 3 A through it for 20 seconds we pass a total of 60 C.
Much of the early work on electrolysis was carried out by Michael Faraday. Faraday’s First Law of Electrolysis states that in electrolysis the mass of a substance liberated is directly proportional to the quantity of electricity provided. Or, put more simply (and more obviously), if we pass a current through copper sulphate solution for two minutes we shall get twice as much copper as we would if we passed the same current for only one minute.
Consider the electrolysis of molten sodium hydroxide, discussed earlier.
4 molecules of sodium hydroxide produce 4 atoms of sodium, 2 molecules of water and 1 molecule of oxygen, so 4 moles of sodium hydroxide produce 4 moles of sodium, 2 moles of water and 1 mole of oxygen molecules. The mole is fully discussed on the Page on Atomic weight and the mole.
Taking the atomic weights of sodium, hydrogen and oxygen to be 23, 1 and 16 160 g of sodium hydroxide produce 92 g of sodium, 36 g of water and 32 g of oxygen.
Now consider what happens at the cathode
We often think of the mole as being concerned only with atomic and molecular weight but we need to remember that the mole is actually the amount of something, anything, which contains 6.02 × 1023 particles.
Here 1 sodium ion and 1 electron make 1 sodium atom, so a mole of sodium ions and a mole of electrons make a mole of sodium atoms. The charge (positive or negative) on a mole of electrons or singly ionised ions such as Na+ is called the Faraday constant. As the charge on an electron is about 1.602 × 10-19 coulombs the Faraday constant is about 9.6 × 104 coulombs/mole. Some people use the Faraday constant as the basis for a unit of charge called the faraday (where one faraday = 9.6 × 104 coulombs) but this has never been officially adopted as a SI unit, and is best avoided as there is already a unit (of capacitance) called the farad.
To make 4 moles of sodium we need 4 moles of electrons, so to make 1 mole (23 g) of sodium we would need a charge of nearly a hundred thousand coulombs. Sodium is very slightly less dense than water so 23 g of sodium would nearly fill an egg-cup. A current of one ampere is one coulomb per second, so if we plugged the equipment into an ordinary 13 A power socket, to make an egg-cupful of sodium we would need to use a kilowatt of electricity (a fan heater full on) for more than six hours!
Extracting metals from their ores by electrolysis needs huge amounts of electricity.

Although aluminium is by far the commonest metal in the Earth’s crust it was also one of the last to be extracted. Sir Humphrey Davy could not make it in the same way that he could make sodium or potassium because aluminium oxide has far too high a melting point. It was first extracted by the Danish scientist Hans Christian Oersted (1777 - 1851) in 1825 by heating anhydrous aluminium chloride with potassium amalgam, and for the next fifty years it was little more than a scientific curiosity, far more expensive than gold.
Then in 1885 two people, Paul Louis Toussaint Heroult in France, and Charles Martin Hall in the United States, quite independently invented the process which is the basis of the method used today.
The main ore used for the production of aluminium is bauxite, aluminium oxide. This has a very high melting point, more than 2000oC. Heroult and Hall quite independently discovered that aluminium oxide can be dissolved in molten cryolite (sodium hexafluoroaluminate, melting point 1016oC), and that this solution is an electrolyte.
Today we contain the melt in a graphite-lined crucible and this acts as the cathode; we use a graphite rod as the anode. When a current is passed through the melt molten aluminium is formed at the cathode. This is denser than molten cryolite so it sinks to the bottom and can be run off at intervals. Oxygen is formed at the anode but at the temperature of the melt this reacts with the carbon from which it is made to form carbon dioxide so the anode is gradually used up.
Quite uniquely, both men were granted patents for the same invention.
Very large quantities of electricity are needed, not only for the electrolysis but also to keep the cryolite molten, and it is only commercially feasible in places with large amounts of hydro-electric power.
During the Second World War much of the aluminium needed by Britain to make fighters and bombers came from aluminium smelters in Canada and the United States of America using electricity from the Niagra Falls hydro-electric power stations. In 1942 Nazi Germany sent two U-boats on a mission to land agents in the United States to sabotage these power stations and so, it was hoped, reduce Britain’s capacity to make military aircraft. This mission however failed to achieve its objective.
Cryolite is not common in the Earth's crust but since 1885 a few other minerals with similar properties have been discovered and these are increasingly used instead of cryolite.
Aluminium is a very reactive element and very quickly reacts with the oxygen in the air to form aluminium oxide. Anything made of aluminium is therefore covered with a very thin coating of aluminium oxide. Unlike rust on iron this does not flake off the surface, so it protects the aluminium from further oxidation, and also water and many other chemicals. So aluminium appears to be very unreactive.
Aluminium windows and many other objects made of aluminium are often anodised. The window is used as the anode in the electrolysis of dilute sulphuric acid using a graphite cathode. Hydrogen is released at the cathode and oxygen at the anode. This oxygen reacts with the aluminium to make a much thicker layer of aluminium oxide, and so much better protection.

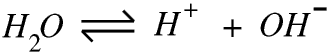
pH stands for potential of hydrogen and the pH scale is a logarithmic scale for measuring the concentration of hydrogen ions in a liquid. For school purposes it is sufficient to consider that a pH of 3 means that there is 1 g (1 mole) of hydrogen ions in every 103 litres of solution.
Pure water is only very slightly ionised: at room temperature there is only 1 g of hydrogen ions in every 107 litres of water, so its pH is 7. This is why very pure water is a very poor conductor of electricity, although tiny amounts of any ionic impurity will greatly increase its conductivity. This is why electricity is so dangerous in a bathroom.
If we take Ar(H+) = 1 and Ar(H2O) = 18 and the density of water to be 1000 g per litre, then if pure water was completely ionised there would be 1 g of hydrogen ions in 0.018 litres of water. But there is not, there is 1 g in every 107 litres, so only one molecule of water in every 107 ÷ 0.018, or 5.56 × 108, is ionised.
In an acid there are more hydrogen ions than in pure water so the volume of solution containing 1 g of hydrogen ions will be less than 107 litres. If there were 1 g in every 105 litres the pH would be 5, a pH of 2 would mean there was 1 g of hydrogen ions in every 102 litres of solution, so the lower the pH the stronger the acid. If you have a good understanding of powers you will realise that the pH of a very strong acid can be zero or even negative.
In an alkali there are fewer hydrogen ions than in pure water so the pH will be more than 7.
The ionisation of pure water is a chemical process and so is affected by temperature. At higher temperatures it is more ionised so there are more hydrogen ions and the pH is lower, even though the ratio of hydrogen ions to hydroxide ions remains 1:1. At 100°C the pH is 6.14.
Very high temperatures can be obtained by focusing the Sun's rays with a parabolic mirror and in experiments water has been separated into hydrogen and oxygen in this way - this has all sorts of very exciting possibilities for the future.
