Carbon is different from all other elements because carbon atoms can join with each other to form rings or long chains. Molecules of compounds of carbon can therefore be much larger and more complex than those not containing carbon.
Compounds consisting of these very large complex molecules are present in all living things, in fact they are what makes life on this planet possible.
At one time it was thought that these very large complex molecules could only be made by living organisms (living things such as animals and plants, bacteria and fungi), so they were named organic compounds, and the study of organic compounds (chemicals) was named organic chemistry. Today however we can make many organic chemicals in laboratories, even factories, so we now use the term organic chemistry to mean the study of compounds of carbon. But carbonates and carbon monoxide and carbon dioxide, which do not contain rings or chains of carbon atoms, are usually classed as inorganic chemicals.
Compounds containing rings of carbon atoms are usually called aromatic compounds, because they often have a strong, usually quite pleasant, smell; compounds containing only chains of carbon atoms are called aliphatic compounds. The rest of this Page is about aliphatic compounds. Organic chemistry often appears very complicated because many organic chemicals have several different names, often with lots of hyphens.
In aliphatic organic compounds each carbon atom is joined with other atoms, of carbon or other elements, by four covalent bonds.
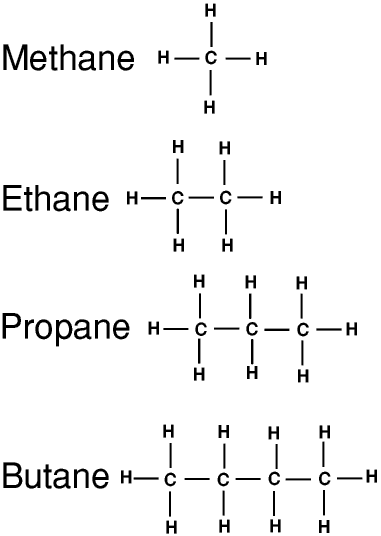
The simplest organic compounds are the alkanes. These are compounds of carbon and hydrogen only: organic compounds containing only carbon and hydrogen atoms are called hydrocarbons

The general formula for all alkanes is CnH(2n+2), so pentane is C5H12, hexane is C6H14, heptane is C7H16, octane is C8H18, and so on.
The alkanes form what is called a homologous series, from the Greek for The Same Rule, and each member of a homologous series is called a homologue. Homologous series are very common in organic chemistry.
If we change one of the hydrogen atoms in the alkane series for an atom of another element or a group of atoms we form a different homologous series. The alcohols for example are formed by substituting an (OH) group for a hydrogen atom. So methanol is CH3OH, ethanol is C2H5OH, propanol is C3H7OH, etc. The “alcohol” in alcoholic drinks is ethanol. The alcohols are described on another Page of this Web Site.
A CH3 group is often called a methyl group, a C2H5 group an ethyl group etc, so methanol is also known as methyl alcohol, ethanol as ethyl alcohol, etc. This naming system leads us on to other compounds such as methyl bromide (or bromomethane) CH3Br, ethyl chloride (or chloroethane) C2H5Cl, etc.
The alkanes are obtained from petroleum and natural gas - this is discussed later on this Page. Methane, ethane, propane and butane are gases at room temperature and pressure- they are called the petroleum gases. Pentane is just a liquid, with a boiling point of about 36oC, hexane and other alkanes with less than 16 carbon atoms are liquids, and those with 16 or more carbon atoms are waxy solids. We normally call alkanes (and all other organic compounds) with only a few carbon atoms and low molecular weights the lower compounds, and those with many carbon atoms and higher molecular weights the higher compounds.
All the alkanes burn but they need a lot of air or oxygen to burn completely, so unless they are deliberately mixed with lots of air first they usually burn with a very smoky yellow flame. Most modern bunsen burners are designed to burn methane (or butane where there is no piped gas supply), hence the air hole. See what happens when you close it. Apart from burning they are not very reactive, hence their old title the paraffins, from the Latin parum affinis, little affinity. The paraffin we use in paraffin heaters is a mixture containing mainly alkanes with 11 or 12 carbon atoms. Petrol contains mainly alkanes with 5 to 8 carbon atoms, and diesel mainly alkanes with 13 to 25 carbon atoms. In diesel fuel, although the alkanes with more than 16 carbon atoms are solids they are dissolved in the lower alkanes. But if you get diesel on your hands or spill any on the ground the lower, liquid, alkanes evaporate leaving behind the higher, solid, ones: this is why you should always wear disposable gloves when filling your car with diesel and why a diesel spill is much more difficult to clean up than a petrol spill, where everything in it evaporates. In very cold weather some more of the lower alkanes in the diesel may become solids and also the solid alkanes are less soluble, so the diesel fuel may become waxy and then you need to use special fuel heaters. The oil companies usually change the composition of their diesel between summer and winter to help overcome this problem.
Natural gas as it comes out of the ground is about 80% methane, with small amounts of ethane, propane and butane and other gases, and also water vapour. This is called wet natural gas. The technical name for the gas that is piped into our homes is dry natural gas, wet natural gas that has been treated to remove almost everything except the methane, including the water, but with a harmless smelly substance added so we know when we have left the gas on or got a leak. The gas in our homes is discussed at greater length on another Page of this Web Site.
Methane is also produced by the action of anaerobic bacteria (bacteria which live without air) on decaying organic matter. Anaerobic bacteria live in many places, including in our intestines, on rubbish tips and in the mud at the bottom of ponds. Vets should not smoke when working near the back end of cows: one did and lost his eyebrows when a sudden jet of methane shot out and caught fire! Rubbish tips have to be provided with some safe way of getting rid of the methane produced, otherwise it might collect and eventually start a huge underground fire: it is often piped to the surface and flared off, but increasingly it is being collected and used as a fuel for heating buildings. Bubbles of methane produced by plants decaying at the bottom of a marsh may also sometimes contain other substances which cause the methane to catch fire spontaneously when it reaches the surface, producing a sudden puff of flame. In the late evening when it is getting dark these puffs of flame are clearly visible. They appear and disappear so quickly at different places all over the surface that in the old days people used to think they were little fairy-like creatures dancing on the surface of the water, hence their name Will o’ the wisp.
Ethane, propane and butane are obtained from wet natural gas by separating them from the methane, and they are also produced in oil refineries. Although propane and butane are gases at room temperature and pressure they can be stored as a liquid under pressure, and this makes them very useful as fuels in places without a piped natural gas supply, on building sites, houses in remote villages, and of course for campers and picnickers and in caravans and boats. They are also being increasingly being used as fuel for cars and lorries. Calor Gas® is propane and Camping Gaz® is butane; LPG (liquefied petroleum gas) is the generic name for both propane and butane. Propane and butane are discussed at greater length on another Page of this Web Site, on the storage of gases.
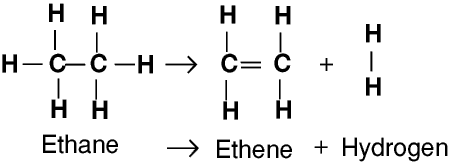
Ethane is not usually used as a fuel because it cannot be stored as a liquid under pressure and so is less convenient to use than propane or butane, but it can be cracked to produce hydrogen and ethene for polymerization - these terms are described below.
The carbon atoms in most alkanes in petroleum are arranged in straight chains, where the carbon atoms are arranged in a single long chain. In books and on web pages we usually draw an alkane molecule as a straight chain (as I have done above), but the molecule is actually three-dimensional, with each carbon atom in the centre of a regular tetrahedron (a shape made out of four equilateral triangles.) You can best understand what follows by making some models. If you are at school your science department almost certainly has a molecular model kit, otherwise you can make your own out of modelling clay and match sticks.
There is only one way to construct a model of a methane or ethane molecule, but more than one way of making propane and several ways of making butane. These different shapes are called isomers, or more correctly stereo-isomers. Stereo-isomers have exactly the same structure of covalent bonds so they all have exactly the same chemical properties, but they do have different shapes and so may have slightly different physical properties. In particular they may have different effects on light, and especially polarised light. Most naturally occuring alkanes are a mixture of several different stereo-isomers; you cannot of course see the different effects they have on light unless you separate them.
It should not surprise us that different stereo-isomers have different effects on light. We see things only because the light reflected off them enters our eyes: an apple and a banana look different to us only because they reflect light in different ways, and they reflect light in different ways only because they are, well, different shapes.
Everything above is about straight-chained molecules, where the carbon atoms are arranged in a single long chain. But they can also be arranged in branched chains. The names of branched chain alkanes are often preceded by iso-
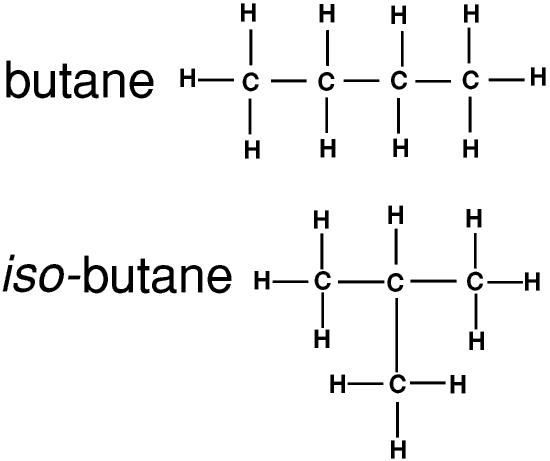
The names of straight-chained alkanes are sometimes prefixed with n-. We say that n-butane and iso-butane are structural isomers: structural isomers have the same chemical composition but different arrangements of the covalent bonds so they do not have exactly the same physical or chemical properties.
This is where organic chemistry begins to appear complicated: iso-butane can also be thought of as propane with one hydrogen atom replaced by a CH3 group so it is also known as methyl propane!
Branched-chain alkanes can be made by cracking higher hydrocarbons - cracking is described later on this Page.

The simplest alkenes are also hydrocarbons, with the general formula CnH2n. They are very similar to the alkanes except that they have a double bond between two of the carbon atoms in the chain. They are called unsaturated hydrocarbons, whereas the alkanes are saturated hydrocarbons.
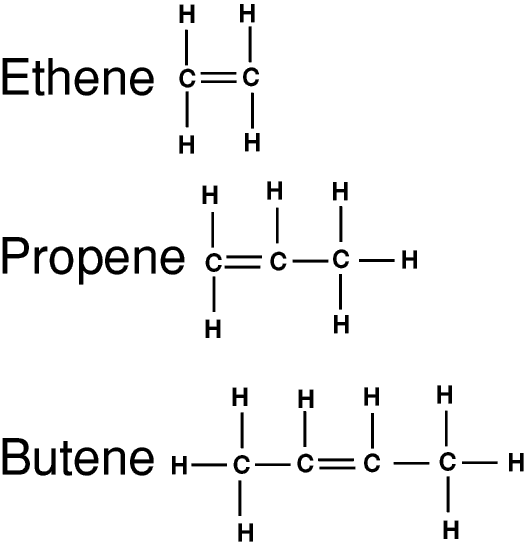
A double bond is not as strong as a single bond, so it is more easily broken. This means that the alkenes are more chemically reactive than the alkanes.
Unsaturated compounds (with double or triple bonds) form additive compounds, where two substances combine to form a single substance. For example ethene reacts with bromine to form dibromoethane.
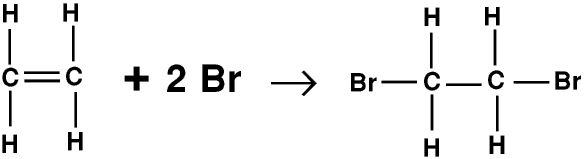
Bromine is a reddish brown liquid, so if any unsaturated compound is shaken up with a solution containing bromine a reaction takes place and the solution goes colourless. This is a standard test for an unsaturated compound.
The alkenes are not as useful as fuels as the alkanes; by far their most important use is in making polymers - this is described in another Section of this Web Page.
Ethene was once called ethylene and propene propylene, and these names are still used in the names of polymers made from them.
There are also other hydrocarbons containing two or more double bonds - these are not considered further on this Page.

The simplest alkynes are unsaturated hydrocarbons with the general formula CnH(2n-2). They are similar to the alkanes and alkenes but have a triple bond and are much more reactive. As for the alkenes, there are also other hydrocarbons with two or more triple bonds. These are not considered further on this Web Page.
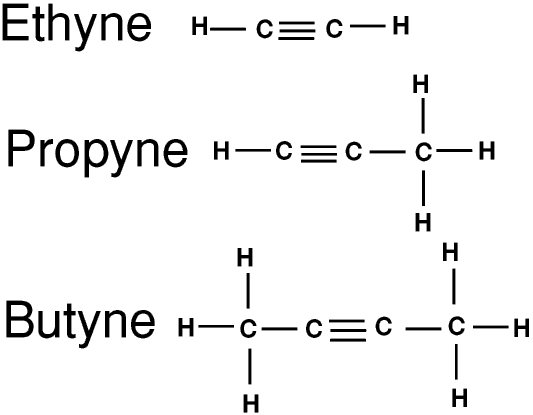
Ethyne is commonly called acetylene. Acetylene burns with a very hot flame, and oxy-acetylene burners are used for many purposes including flame-cutting (using a needle-shaped flame to cut through metal). Oxy-acetylene burners can even be used under water(!) so they are used for cutting up sunken wrecks.
Acetylene is produced when calcium carbide reacts with water.
It was obviously very dangerous to use oil or gas lights when you were filling your fuel tank with petrol, so there was a Law which said petrol filling stations had to put up a large sign telling you to put them out. This Law has never been repealed, so next time you fill up your car with petrol look for a large sign which says “Extinguish all lights oil and gas.”

Petroleum is a very complex mixture of hydrocarbons and other organic chemicals, but consisting mainly of straight-chained alkanes. (Petroleum, from the Greek for oil from rocks, is the correct term although crude oil or even just oil are also very commonly used.)
The lower alkanes (except ethane) in petroleum can be used as fuels (methane and LPG, petrol, paraffin, aviation fuel, diesel, central heating oil, fuel oil for ships and power stations etc).
Ethane, the higher alkanes and other hydrocarbons are not used as fuels, but they have many other uses, and they can also be cracked. When a higher hydrocarbon is cracked it may be turned into two lower hydrocarbons, often one alkane and one alkene, but always at least one unsaturated hydrocarbon. The alkane may be used as a fuel or cracked again, and the alkene may be made into a polymer (see below). Cracking (and another related process called reforming) can also be used to produce branched-chain alkanes. Cracking can be carried out by heating the hydrocarbon or in a catalytic (or cat) cracker.
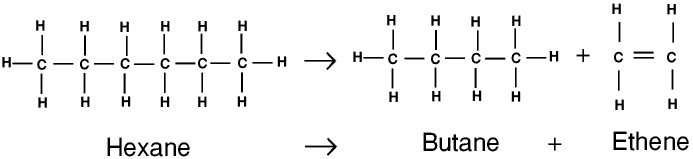

Under certain conditions many simple organic molecules can join themselves together into very much larger molecules. This is called polymerization, and the giant molecules produced are called polymers. Molecules which can be polymerized are called monomers.
The simplest form of polymerization is called additive polymerization. The general reaction is

where M is a monomer and n is typically several hundred. Monomers involved in additive polymerization are alkenes and other unsaturated molecules.
Some additive polymers areCondensation polymers are slightly more complicated as one polymer is formed from two different monomers. Nylon is a condensation polymer. Starches and proteins are naturally occuring condensation polymers.
