Oxidation and reduction were originally used to describe only chemical reactions involving oxygen, but over the years the meaning of these words has been extended to cover many other reactions. Students studying chemistry usually start with the original meaning, which I shall call the traditional meaning. The current meaning is much broader. If you are studying chemistry beyond normal school leaving age or earning your living by it you will certainly need to understand it, but for many people and for many purposes the traditional meaning is sufficient.
Actually I do not think that it is possible to regard the current meaning as merely a logical extension of the traditional meaning: to me it is more like a total rewrite. For this reason I have considered the two meanings quite separately.
There is in fact nothing wrong with using the same word to mean different things: we do it all the time, for example “A day is twenty four hours but the days are longer in the summer than the winter.”
The magnesium has combined with oxygen. We call this reaction an oxidation and we say the magnesium has been oxidised.
If we burn natural gas (methane) in air this reaction takes place
Here the carbon and the hydrogen have each been oxidised.
The oxygen for the oxidation needs to come from somewhere. We call the source of the oxygen the oxidising agent or oxidant. In these two reactions the oxidant is the air. On the surface of the Earth air surrounds us so it is almost always freely available but it is not a particularly good oxidant because air is only about 20% oxygen and has a very low density. For example if we burn carbon in air this reaction takes place.A mole of carbon atoms needs a mole of oxygen molecules, and this will have a volume of about 24 litres, so to burn a mole of carbon (12 g or about a small handful of barbeque charcoal) we need more than 120 litres of air! The mole is more fully explained on the Page on Atomic weight and the mole.
Most liquid fuel rockets, from the Second World War V2 to the Space Shuttle, burn their fuel using liquid oxygen as the oxidant. The oxidant used in solid fuel rockets, including the booster rockets on the Space Shuttle, is described later on this Page.
If we heat copper oxide powder in hydrogen this reaction takes place
The hydrogen has been oxidised, and the copper oxide is the oxidant. The copper oxide has given up its oxygen: we say it has been reduced, and the process has involved a reduction. Traditionally oxidation is when a substance gains oxygen and reduction is when it loses oxygen.
To reduce a substance we need a reducing agent or reductant: here the reductant is the hydrogen. The reaction between copper oxide and hydrogen involves both a reduction and an oxidation: we call it a redox reaction. Many redox reactions are highly exothermic (they give out a lot of heat). A redox reaction involves an oxidant and a reductant, the oxidant is reduced and the reductant is oxidised!
Remember that it is the copper oxide that is reduced: in exams it is a very common mistake to say that it is the copper that has been reduced.
We can arrange metals in an order of reactivity. Here is the list for some common metals.
A metal higher on the list will reduce the oxide of a metal lower on the list, for example if we have a mixture of aluminium powder and iron oxide powder and light it with a short piece of magnesium ribbon as a fuse this reaction takes place
This is called the “thermit” reaction. So much heat is given out that the iron produced is in the form of molten iron. It has been used as a way of producing molten iron for emergency repairs to railway lines in very remote places.
Hydrogen and carbon are very important industrial reducing agents. Hydrogen is manufactured by extracting it from water, and carbon is used in the form of charcoal, made from wood, or coke, made from coal. Wood and coal cannot be used directly because they contain too many other substances.
In the Order of Reactivity hydrogen and carbon are usually placed between iron and zinc. This means they can be used to reduce the oxides of metals below them. Most metals below them are extracted from their ores by heating the oxide with carbon: this process is called “smelting”. This is discussed in greater detail on the Page on Metals and Alloys.
For more than eight thousand years charcoal was used, which meant that all metals were very expensive; the Industrial Revolution began in 1709 when an English Quaker called Abraham Derby first made iron by smelting the ore with coke rather than charcoal and so greatly reduced the cost of iron (also saved Britain’s forests).
Metals above carbon will burn in carbon dioxide, most metals above hydrogen will react with water. For reasons not explained here aluminium does not usually react with water; magnesium reacts slowly with cold water but much more rapidly with steam; calcium reacts vigorously with cold water and sodium and potassium react violently. This means that fires involving magnesium and metals above it cannot be put out with water (or carbon dioxide).
Matches as we know them today were first used at the beginning of the 19th century. They were commonly called Vestas. People bought them loose and kept them in special Vesta cases. The head of the match contained a mixture of an oxidant and a reductant. When the head was rubbed against a rough surface the friction produced enough heat to start the reaction, and the reaction produced enough heat to set fire to the main body of the match. The Vesta case included a rough surface. Vesta matches are still sold, but only in boxes, in Britain under the trade name Swan. They have red heads so they are often called redhead, or strike anywhere matches. They have a piece of sandpaper on the box, but any rough surface will do, a brick wall for example or even, in a television commercial for a new type of electric razor, a man's face.
Any friction might produce enough heat to ignite a Vesta match, even the matches rubbing together in the box. Most modern matches are safety matches: the reductant is put on the head of the match and the oxidant on the side of the box. The match can therefore only be lit by striking it on the side of the box. If you look at the side of the box after only a few matches have been used you can see the marks where the oxidant has actually been used up.
The earliest explosives were mixtures of solid oxidants and solid reductants which reacted together to produce a gas. Gunpowder is a mixture of charcoal, sulphur and potassium nitrate (saltpetre). It was invented by the Chinese in the seventh century CE. The reaction between them causes the very rapid release of a large volume of carbon dioxide and sulphur dioxide gas. It can be used in a cannon to fire a cannonball, in a tube open at one end to act as a rocket, or in a barrel as a bomb.
Solid fuel rockets have been and still are used for very many purposes, from fireworks to anti-tank missiles. They are however not suitable for all purposes because once the reaction has started it continues until all the reactants have been used up - there is no way of controlling it. Liquid fuel rockets almost always use a liquid fuel (reductant) and a liquid oxidant which are stored in separate tanks and pumped in a totally controllable way into a combustion chamber. Described like this it sounds very easy to make one but it is in fact incredibly difficult. The first liquid fuel rocket was the German V2, used against Britain during the last few months of the Second World War. The fuel (reductant) was ethanol and the oxidant liquid oxygen. British Intelligence was receiving lots of reports about the development of the V2 but British scientists could not believe the Germans had overcome the technical difficulties in making a liquid fuel rocket and so did not take them seriously - until the first rockets actually started landing on Britain! Today different fuels may be used but the oxidant is still usually liquid oxygen.
During the last stages of the War the Germans also built a rocket-powered fighter, the Komet. This was powered by a single liquid, concentrated hydrogen peroxide, H2O2. In the presence of a catalyst this decomposed to form oxygen and superheated steam.
During the 20th century many new explosives were developed based not upon a mixture of an oxidant and reductant but upon just a single substance, usually a compound of nitrogen, for example nitroglycerine or trinitrotoluene (TNT). A car airbag system contains sodium azine, NaN3, which when detonated decomposes very rapidly to produce nitrogen and sodium. The nitrogen inflates the airbag, but the sodium is very dangerous so the airbag system contains not only sodium azine but also potassium nitrate and silicon dioxide which react with the sodium in a very complicated way to make it safe.
Oxidation and reduction were originally used to describe only chemical reactions involving oxygen, and this is the meaning used in the above Section. Then the meaning was extended to include reactions involving hydrogen: oxidation included reactions involving the loss of hydrogen and reduction included reactions involving the gain of hydrogen. You may come across these meanings in older organic chemistry books. But then the meaning was extended even further: today oxidation and reduction are concerned with changes in the arrangement of electrons in the electron shells around the nucleus of an atom, that is, all chemical reactions. Some people, including myself and once you have read the whole of this Page probably you, would see this not as an extension of the traditional meaning but as a total rewrite.
Consider the reaction
We say that the sodium has been oxidised and the chlorine has been reduced. In reactions involving ions oxidation involves loss of electrons and reduction involves gain of electrons.
An oxidising agent accepts electrons from another reactant, and a reducing agent donates electrons to another reactant. In general, metals are reducing agents and non-metals are oxidising agents.
When a metal or other element is oxidised its oxidation number is increased. Sodium uncombined with other elements has an oxidation number of zero and positively charged sodium ions have an oxidation of +1.
Similarly when a non-metal is reduced its oxidation number is reduced. Chlorine uncombined with other elements has an oxidation number of zero, and negatively charged chloride ions have an oxidation number of -1.
All elements and ions have oxidation numbers. For any element uncombined with other elements the oxidation number is zero, for any simple ion the oxidation number is the charge.
What this means is, when sodium (or any other Group I element) is combined with another element in a compound it has an oxidation number of +1, and when chlorine (or any other Group VII element) is combined with another element in a compound it has an oxidation number of -1.
We can rewrite the equation for the formation of sodium chloride showing the oxidation numbers.
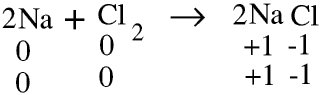
For reasons explained below we show the oxidation number for each atom or ion separately. The oxidation numbers must balance in exactly the same way that the numbers of each atom, the total charge and the total mass must.
For any molecule or compound the sum of all the oxidation numbers of all the elements in it is always zero, for any ion it is the charge on the ion.
The five gases which are very reactive elements (hydrogen, oxygen, nitrogen, fluorine and chlorine) exist in Nature only as diatomic molecules, for example H2. The sum of the oxidation numbers of the two hydrogens in the molecule is zero. As the two hydrogens in it are the same they must have the same oxidation number so this must be zero - the oxidation number of an element only changes when it becomes part of an ion or a compound with another element. Similarly for all the other diatomic gases. In fact, the oxidation for any uncombined element is always zero whatever the allotrope, for example carbon exists as graphite, diamond, graphene and of course “Bucky Balls” but all forms have an oxidation number of zero. Many other elements, from metals like tin to non-metals like sulphur exist as several allotropes.

When carbon burns completely in air it forms carbon dioxide, CO2. The sum of all the oxidation numbers in a compound is always zero. If the oxidation number of combined oxygen is -2 the oxidation number of combined carbon must be +4.
Summarising the basic rules for oxidation numbers
The transition elements have more than one oxidation number, for example mercury has two chlorides, HgCl and HgCl2 These were once called mercurous chloride and mercuric chloride but are now called mercury(I) chloride and mercury(II) chloride, where the number in brackets is the oxidation number. Oxidation numbers for elements with more than one are always shown in this way in Roman numerals, for example iron(II) oxide FeO and iron(III) oxide Fe2O3.
Under certain conditions oxygen forms compounds called peroxides, where the oxygen has an oxidation number of -1. The best known of these is hydrogen peroxide, H2O2, but there are others, for example barium peroxide, BaO2.
Hydrogen peroxide is a clear colourless liquid. In the presence of a catalyst it decomposes to form water and oxygen. Concentrated hydrogen peroxide (high test peroxide or HTP) decomposes so violently that the water is in the form of superheated steam, and this has been used as a rocket fuel. Dilute hydrogen peroxide is used as a bleach.
This Section is intended only as an introduction to the basic ideas behind the current meanings of oxidation and reduction. To take it further you need a detailed knowledge and understanding of electron shells, and s, p, d and f orbitals, and for me to provide this information on this Page would put it beyond my target readership which is essentially non-specialist. There are however many other web pages which do give this information.