An element consists entirely of atoms of the same sort (with the same atomic number - atomic number is explained on my Atomic Structure web page). It cannot be separated into anything simpler by chemical means. There are about ninety elements that occur naturally in the Earth's crust, and several more that have been made by Man in nuclear reactions.
Each element has an internationally agreed symbol consisting of a capital letter or a capital letter followed by a small letter, and on this Page these are given in brackets after the name of the element. This list uses the internationally agreed spellings, but also gives recognised alternative spellings.
Elements are usually arranged in a particular way in a special table called the Periodic Table. You can find out more about the elements on this page, and all other elements, by clicking on its symbol on this Periodic Table web site. Once you start studying chemistry seriously the Periodic Table is a quite indispensible tool, but you do not need to know about it to understand this Web Page.
There are about seventy seven elements which are solids at normal temperatures. Solid elements can be divided into metals, non-metals, and metalloids or semi-metals
![]() Aluminium (Al) (or aluminum, pronounced aloo minum, in the United States)
Aluminium (Al) (or aluminum, pronounced aloo minum, in the United States)
![]() Barium (Ba)
Barium (Ba)
![]() Cadmium (Cd)
Cadmium (Cd)
![]() Calcium (Ca)
Calcium (Ca)
![]() Caesium (Cs) (or cesium in the United States)
Caesium (Cs) (or cesium in the United States)![]() Chromium (Cr)
Chromium (Cr)
![]() Cobalt (Co)
Cobalt (Co)
![]() Copper (Cu, from the Latin cuprum)
Copper (Cu, from the Latin cuprum)
![]() Gallium (Ga)
Gallium (Ga)![]() Gold (Au, from the Latin aurum)
Gold (Au, from the Latin aurum)
![]() Iron (Fe, from the Latin ferrum)
Iron (Fe, from the Latin ferrum)
![]() Lead (Pb, from the Latin plumbum)
Lead (Pb, from the Latin plumbum)
![]() Lithium (Li)
Lithium (Li)
![]() Magnesium (Mg)
Magnesium (Mg)
![]() Manganese (Mn)
Manganese (Mn)
![]() Nickel (Ni)
Nickel (Ni)
![]() Platinum (Pt)
Platinum (Pt)
![]() Polonium (Po)
Polonium (Po)![]() Potassium (K, from the Arabic kalium)
Potassium (K, from the Arabic kalium)
![]() Radium (Ra)
Radium (Ra)
![]() Silver (Ag, from the Latin argentum)
Silver (Ag, from the Latin argentum)
![]() Sodium (Na, from the Latin natrium)
Sodium (Na, from the Latin natrium)
![]() Tin (Sn, from the Latin stannum)
Tin (Sn, from the Latin stannum)
![]() Titanium (Ti)
Titanium (Ti)
![]() Tungsten (W, from the Old English wolfram)
Tungsten (W, from the Old English wolfram)
![]() Uranium (U)
Uranium (U)
![]() Zinc (Zn)
Zinc (Zn)
There are only two elements that are normally liquids:
![]() Bromine (Br)
- a non-metal
Bromine (Br)
- a non-metal ![]() (Melting point -7oC, Boiling point 58oC)
(Melting point -7oC, Boiling point 58oC)
![]() Mercury (Hg, from the Greek hydrargyrum meaning liquid silver)
- a metal
Mercury (Hg, from the Greek hydrargyrum meaning liquid silver)
- a metal ![]() (Melting point -39oC, Boiling point 357oC
)
(Melting point -39oC, Boiling point 357oC
)
There are eleven elements which are gases. There are eight gaseous elements which are present in the atmosphere:
![]() Oxygen (O)
Oxygen (O)
![]() Nitrogen (N)
Nitrogen (N)
![]() The
noble (or rare or inert) gases:
The
noble (or rare or inert) gases:
For more about the gases in the atmosphere please visit my Web Page on Atmospheric Gases.
The noble gases exist in the atmosphere as single atoms: we call them monatomic gases. All the other gases which are elements, that is, hydrogen, oxygen. nitrogen, fluorine and chlorine, exist as diatomic molecules, containing two atoms, for example H2.Some elements can exist in two or more forms depending on the way the atoms are arranged or combined together: these forms are called allotropes. For example graphite and diamond are two forms of the element carbon. Oxygen has two allotropes, normal oxygen (O2) and ozone (O3).
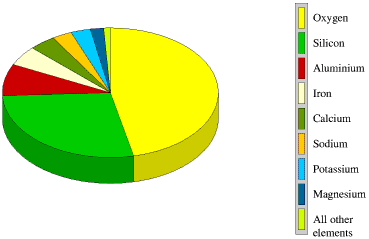
The approximate proportions of the elements in the Earth's crust are
![]() oxygen 47%
oxygen 47%
![]() silicon 28%
silicon 28%
![]() aluminium
8%
aluminium
8%
![]() iron 5%
iron 5%
![]() calcium 4%
calcium 4%
![]() sodium
3%
sodium
3%
![]() potassium 3%
potassium 3%
![]() magnesium 2%
magnesium 2%
All other
elements together make up less than 1% of the Earth's crust.
The Earth’s core, and therefore the Earth as a whole, is mostly iron but the crust is the only part accessible to Man.
© Barry Gray July 2016