Calcium carbonate is a very common mineral in the Earth's crust. It is the main building block of most animal shells, including the shells of shellfish, snails and birds’ eggs.
There are four main types of rock containing calcium carbonate.
Limestone is a sedimentary rock, formed from the fossilised remains of animal shells. It is found in most parts of the world. It is easily cut and shaped and so is often used for building. The Pyramids of Ancient Egypt and many of the great mediaeval cathedrals of Britain and Europe are built mainly of limestone.
Most limestone was formed hundreds of millions of years ago in ocean sediments - this is discussed further later on this Page.
Marble is metamorphic limestone, limestone that has been changed by very high temperatures and pressures. It occurs mainly in areas of high geological activity, where there are lots of volcanos and earthquakes. It is therefore very common in the Northern Mediterranean, which is why the Greek and Roman temples were built of marble.
Chalk is the crushed but not yet fossilised remains of the shells of tiny marine animals - sometimes you can find tiny pieces of shell in it that have not been completely reduced to powder. The North and South Downs of Southern England, and of course the White Cliffs of Dover, are huge chalk deposits. Most chalk deposits were laid down in relatively shallow water during the cretaceous period, about 150 to 65 million years ago. (In fact, cretaceous comes from the Greek for chalk.)
It should be noted that “blackboard chalks” are made of calcium sulphate not calcium carbonate.
We often refer to any powdery form of calcium carbonate as chalk, for example the “chalkiness” in hard water after it has been boiled - this is discussed later on this Web Page - and also often any powdery substance, for example we may say that some indigestion tablets taste a bit chalky.
Calcite is the name given to a group of crystaline forms of calcium carbonate. One form of calcite is Iceland Spar. This forms very beautiful clear colourless crystals. They are very easily cleaved (split along definite planes) and so are often used in schools to demonstrate this. They also refract light in two different ways to produce a double image. You can see some pictures of calcite crystals, and the double image produced by Iceland Spar crystals, by visiting the calcite website - to do so please click here ![]()
Limestone is by far the most important source of calcium carbonate for both biological and Man-made processes. Limestone has many very important uses, and these are discussed in the last Section of this Web Page.
For the rest of this Web Page we shall refer mainly to limestone, although the other forms of calcium carbonate usually behave in a chemically similar way.
Calcium oxide is known as lime, or sometimes quicklime. If we heat a lump of quicklime very strongly it gives out a very bright white light. This is known as limelight. Limelight was used for stage lighting before the introduction of electricity, so famous actors and actresses were said to be in the limelight.
Quicklime reacts very violently with water giving out a lot of heat.
This reaction is called slaking. Calcium hydroxide is also known as lime, or sometimes slaked lime.
Because of the amount of heat given out in this reaction, sometimes the bodies of people who had died of disease, particularly bubonic plague, were covered in quicklime before they were buried: it was thought that the high temperatures reached as the lime became damp would destroy whatever it was that was causing the disease.
Calcium hydroxide dissolves in water to form a slightly alkaline solution. A saturated solution of calcium hydroxide in water is called lime water; if we add more calcium hydroxide and keep the mixture well shaken we have a suspension of calcium hydroxide in water which is called milk of lime. Milk of lime was at one time used as a whitewash for walls. It was also sometimes used to treat indigestion and other stomach complaints, but today milk of magnesia (a suspension of magnesium hydroxide in water) is more commonly used.
As slaked lime is alkaline it is often used on farms and in gardens to treat soils which have become acidic, although it is not a fertiliser.
Calcium hydroxide has been used to make lime mortar and lime plaster for buildings for thousands of years - lime mortar was used in the Pyramids of Ancient Egypt. The lime needed is usually made from limestone but can also be made from any other form of calcium carbonate. The Mausoleum at Halicarnasus was one of the Seven Wonders of the Ancient World. It was built in 353 BCE as the tomb, a very elaborate tomb indeed, made largely of marble, for King Maussollos (which is why today we use the word mausoleum for any very elaborate tomb). Halicarnasus is in modern Turkey; today the town is called Bodrum. The Mausoleum was still almost intact more than a thousand years later. Then in 1494 the Crusaders needed mortar to repair the castle they had built at Bodrum, and used the marble from the Mausoleum to make their lime. Within a few years there was nothing left of it....
Lime mortar hardens as it dries. In addition a chemical reaction takes place between the lime and the carbon dioxide in the air.Calcium carbonate does not dissolve in water but, like all carbonates, it reacts with acids.
All carbonates (and also hydrogencarbonates - see below) give off carbon dioxide when they are treated with an acid. This is one of the standard tests for a carbonate, and is also a way of producing small amounts of carbon dioxide in the laboratory and to power toys. You can buy jet-propelled boats and other toys powered by the carbon dioxide given off when vinegar (ethanoic acid) is added to bicarbonate of soda (sodium hydrogencarbonate).
Carbon dioxide dissolves in water to form a weak acid called carbonic acid.
This reaction is reversible. As air contains small amounts of carbon dioxide rain water is naturally very slightly acidic (about pH 6) - but this must not be confused with acid rain which is due to Man-made pollution and is very strongly acidic (pH 2 or 3). Acid rain is discussed on the Page on Atmospheric Pollution.
Calcium carbonate reacts with carbonic acid to form calcium hydrogencarbonate (calcium bicarbonate). This reaction is reversible.Calcium hydrogencarbonate is slightly soluble in water. This means that limestone dissolves very slowly in rainwater.
In limestone areas the underground water may seep through cracks in the rock gradually dissolving away the rock leaving very complex systems of interconnecting underground caves. Some of these caves may be flooded with water and some may be dry or have water only at the bottom. If water drips slowly from the roof of an unflooded cave, as the drop hangs from the roof a little of the water may evaporate and a little of the carbon dioxide dissolved in it will be returned to the atmosphere, so a minute amount of calcium hydrogencarbonate will be decomposed to form insoluble calcium carbonate, in its crystaline form of calcite.
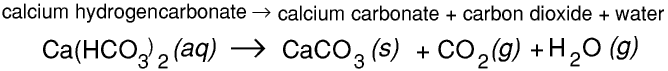
Stalactites grow down from the roof of the cave. If the cave is absolutely draught-free, and nothing at all prevents the drops of water falling from the stalactite from landing on exactly the same spot on the floor of the cave for hundreds of years, a stalagmite may grow up from the floor of the cave. If they meet we have a column.
You can read more about this, and see some wonderful photographs of stalactites, by visiting a Cave Photographs web site - to link to one please click here ![]()
If we heat water, for example hard tap water (see below), containing dissolved calcium hydrogencarbonate, carbon dioxide, like all other gases, is less soluble in hot water than cold water, and some of the carbon dioxide is driven out of the water, the calcium hydrogen carbonate decomposes, and insoluble calcium carbonate is formed. The chemistry is as for the formation of a stalactite, except that the calcium carbonate appears as a chalkiness in the water rather than forming a calcite crystal. This is the cause of chalk inside kettles in hard water areas - hard water is discussed later in this Web Page.
If we bubble carbon dioxide, or a mixture of gases which includes carbon dioxide such as air or our breath, through lime water calcium carbonate is formed in suspension and the lime water goes milky.
Initially the lime water is alkaline. As we bubble more carbon dioxide through it more dissolves and eventually the solution becomes acidic. At this point the solution starts to become clear again due to the formation of calcium hydrogencarbonate.
Almost all living things need calcium. As described above animal shells are made mainly of calcium carbonate; the bones of mammals and other vertebrates are made mainly of calcium phosphate. On land, almost all calcium enters the food chain in the form of calcium hydrogencarbonate formed by the action of rainwater on limestone: this dissolves in the water and is taken up by plants. Huge quantities of carbon dioxide are dissolved in the seas so seawater is very slightly acidic and calcium enters the food chain in the seas in almost the same way.
Calcium carbonate (and calcium phosphate) is not broken down by biological processes, so shells and bones do not decompose. On land the big bones are normally broken up by scavengers in order to get at the marrow, what is left will usually get crushed and buried, or be dissolved by rainwater. In the seas and oceans the calcium carbonate in the shells of crustacea and other animals will sink to the bottom and form a sediment, taking with them not only the calcium but also the carbonate. Huge quantities of carbon dioxide are locked up in ocean sediments, and the removal of carbon dioxide from the oceans, and therefore also from the atmosphere, in this way was a key factor in the changes in the composition of the atmosphere which allowed most of today's life forms to evolve.
There are two sorts of hardness: temporary and permanent. Both produce this scum and reduce the efectiveness of soaps and detergents.
Temporary hardness is due to dissolved calcium (or magnesium) hydrogencarbonate. If this water is heated the carbon dioxide is driven out of solution, the calcium hydrogencarbonate decomposes and the water goes cloudy as calcium carbonate (chalk) is left in suspension. This then forms limescale inside kettles, washing machines and dishwashers, hot water pipes and water heaters etc. However, once the water has been boiled and the chalk allowed to settle the hardness is removed - hence the term “temporary” hardness.
Permanent hardness is due to dissolved calcium (or magnesium) sulphate. This is not decomposed by heating and so it does not leave limescale inside kettles, pipes or water heaters etc, but it is not removed by boiling the water, hence the term “permanent” hardness.
Tap water may contain naturally occurring calcium and magnesium compounds, and, depending on where it has come from, it may also contain small amounts of other substances, including Man-made pollutants such as lead. In most countries chlorine is also added to the drinking water to kill germs. Drinking water filters (such as “Brita” and similar) use a cartridge containing special chemicals which absorb or react with some of the substances in tap water, and so reduce the amount of chlorine and other gases, heavy metals such as lead, and calcium and magnesium compounds. When a certain amount of water has been treated the cartridge must be thrown away and replaced.
Chemical water softeners work in a different way. The water from the mains passes through a special resin containing compounds of sodium. These react with compounds of calcium and magnesium: calcium and magnesium ions are replaced with sodium ions. Sodium compounds are not decomposed when the water is heated, and do not form a scum when mixed with soap, so the water is soft. After a time all the sodium in the resin has been replaced with calcium and magnesium and it must be regenerated: this consists of passing brine (a saturated sodium chloride solution) backwards through it.
Water softened in this way can be use for washing, and in washing machines and water heaters etc, but it contains compounds of sodium and so should not be used for drinking, particularly by children or old people or those with heart disease. If you have a chemical water softener you should have a separate hard water tap for drinking water. (Also for the garden.)
De-ionisers work in yet a different way. They remove all the dissolved solids from the water and are used for making “purified water” for use in science and medical laboratories. They are not considered further here.Cement is made by heating a mixture of limestone and clay and other substances. The cement can then be mixed with other substances to make cement mortar and concrete. The chemical reactions involved in the manufacture and use of cement, cement mortar and concrete are not considered further here. The Greeks and Romans knew how to make and use cement and concrete; the Romans even discovered how to make a type of concrete which sets under water. Today huge amounts of cement and concrete are used by the building industry, and limestone is quarried on a very large scale to make them. You can read more about this by visiting the British Cement Association web site.
Limestone is also used in the production of iron. It is added to the iron ore and coke at the top of the blast furnace, and reacts with the impurities in the iron ore to form a slag. This is further discussed on the Page on Ironmaking.
Limestone is also used in the manufacture of glass. Limestone, sodium carbonate and sand (silicon dioxide) are heated together. Sodium and calcium silicates are formed and these cool to form glass. Other substances may also be added to make different types of glass, for example Pyrex, lead glass and crystal, or to colour it.
Limestone is also used in the manufacture of many other chemicals including sodium carbonate (washing soda) and sodium hydrogencarbonate (bicarbonate of soda), and calcium carbide.
The manufacture of cement, and many other industrial processes involving calcium carbonate, produces carbon dioxide and therefore contributes to global warming.