
This Page is one of several Pages about nuclear science. This Page is about nuclear fission; other Pages deal with the structure of the atom, radioactivity, half-life, ionizing radiation and health and safety, uses of radioactivity, carbon and other forms of dating, and nuclear fusion.
This Page assumes you are familiar with the structure of the atom and with radioactivity.
Uranium exists in Nature in the form of two main isotopes: uranium-238 and uranium-235. Both are radioactive, but uranium-235 is also fissile: a uranium-235 nucleus can split into two, forming two new nuclides and producing two or more neutrons. Nuclear fission produces far more energy than radioactive decay. Nuclear power stations and nuclear weapons use the energy released by nuclear fission. Nuclear fission also produces many nuclides, many highly radioactive, which do not normally exist in Nature.
Uranium-235 is the only naturally occurring fissile nuclide, but plutonium-239 and uranium-233 can be produced inside nuclear reactors and these are also fissile. These are discussed later.This Page is about nuclear power and nuclear weapons, and the nuclear industries which have sprung up around them. It is deliberately vague on some matters, and there is a good for this.
In 1995 an American Boy Scout built a nuclear reactor in his back garden, using only information and materials freely available. You can read about this by looking at another web site, on the Atomic Boy Scout
If you want to build a nuclear reactor in your back garden I am sure you can still find all the information you need and order all the materials you need on the Web, but I do not want anybody, you or anyone else, to be able to say I helped you....

Uranium-235 is fissile, which means that the nucleus can split in two, forming two new nuclides with lower atomic numbers and releasing two or more neutrons. Fission can sometimes occur spontaneously, but it more usually occurs when a uranium-235 nucleus is hit by a neutron. When a uranium-235 nucleus is hit by a neutron this is one of the reactions which may occur.
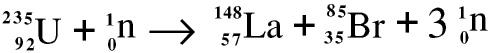
The atomic number and nucleon number are conserved. But when we are dealing with the energy released in nuclear reactions it is not good enough to take the relative atomic mass of a nuclide to be its nucleon number, we must use the actual relative atomic mass. Here they are - but you will not be expected to remember them! They are 147.9321912 for lanthanum-148, 84.91651 for bromine-85, 235.0439242 for uranium-235, and 1.00879 for a neutron. So in this reaction there is a loss in mass of about 0.177 atomic mass units, leading to a release of energy according to Einstein’s famous equation E = mc2. Atomic mass units are discussed in greater detail on the Page on atomic structure.
Lanthanum-148 and bromine-85 do not occur in Nature; they are both radioactive, lanthanum-148 with a half-life of 1.28 seconds and bromine-85 with a half-life of 2.87 minutes.There are several other ways a uranium-235 nucleus may split, for example
But all ways produce two new nuclides, usually radionuclides with very short half-lives which do not occur in Nature, two or more neutrons, and about the same amount of energy. The amount of energy produced is enormous: the fission of one kilogram of uranium-235 produces as much energy as the burning of three million tonnes of coal.
About 85% of of the uranium-235 nuclei which are hit by a neutron will split, releasing two or more neutrons. If these neutrons then cause the fission of other uranium-235 nuclei a chain reaction will occur. Both nuclear reactors and nuclear weapons require a chain reaction to take place; in a nuclear reactor the chain reaction is very carefully controlled, whereas in a nuclear weapon the chain reaction is totally uncontrolled.
Chain reactions do not usually occur in Nature because most of those rocks which do contain uranium contain it only in very low concentrations, and also because today naturally-occurring uranium is about 99.3% uranium-238 and only 0.7% uranium-235, and most of the neutrons produced by the fission of the uranium-235 will escape from the uranium or be absorbed by the uranium-238. For a chain reaction to occur we need to increase the probability that some of the neutrons produced by the fission of one uranium-235 nuclide will cause the fission of another.
The first thing to do is to concentrate the uranium by separating it from the surrounding rocks - exactly as for any other metal. Uranium is present in many rocks, and also seawater. One of the main ores is pitchblende, which contains uranium oxide, and this is mined in several countries including the United States and Canada, Russia, Africa and Australia. After the uranium oxide has been extracted from the rock the tailings (material left behind) contain all the decay products from the uranium-238 and uranium-235 and are very highly radioactive. These decay products are described on this Page.
Now there are two things we can do. One of them is to enrich the uranium. This involves removing some of the uranium-238, so increasing the ratio of uranium-235 to uranium-238. This process requires very large and very specialised equipment.Nuclear weapons must use highly enriched uranium (HEU) containing at least 80% uranium-235, and so some Governments make the assumption that countries that are building facilities to enrich uranium are developing nuclear weapons, although in fact enriched uranium is needed for many purely peaceful purposes - most nuclear power stations need the uranium to be enriched to contain more than 3% uranium-235. The problem with enriching uranium is that for every kilogram of enriched uranium (containing more than 0.7% uranium-235) you produce you produce many more kilograms of depleted uranium (containing less than 0.7% uranium-235), and this is both dangerous and almost completely useless. This is further discussed on another Page of this Web Site - to link to it please click here

Nuclear weapons must use HEU. However this by itself is not enough to produce a chain reaction if the amount of uranium-235 is too small or it is in the form of, say, a long thin wire, so that most of the neutrons will escape from it before they have time to hit another uranium-235 nucleus. For a chain reaction to take place we must have a compact lump, a sphere is ideal, of uranium-235 of a certain minimum mass, the critical mass - this is about 15 kg.
A uranium atom bomb consists of two (or more) lumps of HEU, each individually less than the critical mass but greater than the critical mass when brought together. The bomb is detonated by bringing the pieces together: an uncontrolled chain reaction results. Very high temperatures are produced, and the resulting very rapid expansion of the air, far faster than the speed of sound, sets up huge shockwaves which cause great damage to buildings over a wide area. The heat itself and the radiation also do great damage to buildings and all living things. Such a weapon is sometimes called a fission bomb.
We can also make a fission bomb from plutonium-239 - as described below plutonium-239 is produced by nuclear power stations.
A hydrogen bomb involves nuclear fusion, which is discussed on another Page of this Web Site. But the very high temperature needed to start the nuclear fusion reaction is produced by detonating a uranium atom bomb. A hydrogen bomb is sometimes called a fission-fusion bomb.
A neutron bomb is a special sort of fission-fusion bomb in which almost all of the energy is released in the form of high-energy neutrons rather than heat. These kill all living things, including all humans, but do not damage buildings, roads or bridges etc, not even fuel, food or water supplies. From the military point of view a neutron bomb is the ideal weapon. No country or organisation has yet claimed to have made a neutron bomb.
A cobalt bomb is a fission bomb mixed with cobalt. Cobalt exists mainly as cobalt-59 which is not radioactive, but when cobalt-59 is bombarded with neutrons, and there are lots of these in an uncontrolled chain reaction, it gains a neutron to become cobalt-60, and cobalt-60 is very radioactive, with a half-life of 5.28 years, and very dangerous to all living things. The atomic explosion scatters the cobalt-60 over a wide area. A cobalt bomb is sometimes called a dirty bomb: one was used in the James Bond film Goldfinger but no one outside Hollywood has ever actually made one.

Nuclear power stations use nuclear reactors. In a nuclear reactor a chain reaction occurs but is very carefully controlled.
There are several different sorts of nuclear reactor. Some use enriched uranium to allow the chain reaction to take place, others use ordinary uranium and a moderator, some use enriched uranium and a moderator. A moderator consists of a substance which slows down neutrons without absorbing them: slower-moving neutrons are much more likely to hit uranium-235 atoms and so cause fission. These slow-moving neutrons are sometimes called hot, or thermal, neutrons, because they have kinetic energies similar to the thermal energy of their surroundings. Graphite and beryllium have been used as moderators.
Reactors which do not use a moderator are called fast reactors, because they involve fast neutrons.
During the Second World War both the Allies (Britain and America) and the Germans were trying to build an atom bomb. They both had to do a lot of research into nuclear physics first, and this meant they had to build a nuclear reactor (originally called an atomic pile).
Water is H2O, and contains mainly hydrogen-1 atoms, with 0.0156% hydrogen-2 atoms. Hydrogen-2 is also known as heavy hydrogen or deuterium and given the symbol D. Heavy water is D2O rather than H2O. The first nuclear reactors had to use heavy water as the moderator. Heavy water is made by separating it from ordinary water, and this process needs huge amounts of electricity. The Germans built their heavy water production plant in Nazi-occupied Norway, where there was plenty of hydro-electric power. The British and Americans made several attempts to destroy this factory because they hoped that by doing so they would prevent Hitler’s scientists from building a nuclear reactor, and so eventually an atom bomb: the film 633 Squadron was about one of these attempts. Since the Second World War nuclear reactor design and construction has changed a lot: ordinary water can be used as a moderator in modern reactors, provided the uranium has been enriched to contain at least 3% uranium-235. But it must be water, not steam.

Huge amounts of very highly radioactive materials were of course produced, but these have been totally contained within the rocks surrounding them: tests have shown that the radioactive material has moved less than ten metres relative to the surrounding rocks in more than a thousand million years!

On purely environmental grounds I have been arguing in favour of Britain building more nuclear power stations for more than thirty years.
Almost all nuclear power stations use the heat produced by nuclear fission in a nuclear reactor to heat water to make steam to drive turbines to generate electricity. In this respect they are different from coal or oil or gas power stations only in the way they produce heat.
The uranium fuel, almost always in the form of pellets of uranium oxide, is contained in tubes of zirconium, sealed at both ends and covered with fins to provide a large surface area for cooling. These are the fuel rods.The fuel rods become very highly radioactive in use so they are lowered into the reactor core and removed from it by remotely controlled robots.
A coolant circulates through the core to remove all the heat generated by the nuclear fission, radioactive decay and other nuclear reactions inside it. Different sorts of reactor use different coolants and moderators. Great Britain was one of the first countries to build nuclear power stations, starting in 1965, and currently has twelve, generating between them about 25% of the country's electrical energy. But of course being first means that most of Great Britain's nuclear power stations are now more than twenty years old and not the latest design. Most commercial power stations built within the past twenty years use pressurised water as the coolant - hence they are called PWR power stations, for pressurised water reactor. The cooling water, pressurised to raise its boiling point so it remains a liquid at temperatures well above 100°C, circulates through the core and so becomes highly radioactive. Because it is so radioactive it is not used to make the steam to drive the turbines, instead it is passed through a heat exchanger. It is the water heated in this heat exchanger which is used to produce the steam which drives the turbines.
The reactor is controlled by long control rods. These are made of a material which stops neutrons, such as boron or cadmium. They can be raised or lowered into the core. To produce power in a controlled way it is necessary that on average only one neutron produced by the fission of a uranium-235 atom causes the fission of another uranium-235 atom. The length of each control rod inside the core is adjusted continuously to ensure this happens. If it is necessary to shut the reactor down the control rods drop completely into the reactor under gravity so instantly stopping all fission.
The whole reactor is enclosed inside a very thick radiation shield, and then this is enclosed in another very thick protective shield, both to contain any leakage of radioactive material in the event of an accident to the reactor and also to protect the reactor from damage by external causes such as crashing aeroplanes or earthquakes.
You can read a good but rather technical account of some of the different types of reactor (Magnox, Advanced Gas Cooled Reactor, Boiling Water Reactor etc and also breeder reactors) and their advantages and disadvantages by visiting another Web Site. This Other Site discusses mainly reactors built in the United States and uses non-metric units.

The fuel rods contain uranium-238 as well as uranium-235. Uranium-238 absorbs neutrons which is why we must enrich the uranium or use a moderator, or both. When uranium-238 absorbs a neutron it becomes uranium-239. This is also radioactive with a half-life of 23 minutes. This undergoes beta decay to form neptonium-239, with a half-life of 2.4 days, and this undergoes beta decay to form plutonium-239. This is radioactive, with a half-life of 24110 years, but it is also fissile. The World’s first atom bomb, dropped on Hiroshima, was a uranium-235 bomb; the second, dropped on Nagasaki,was a plutonium-239 bomb. Plutonium-239 is a by-product of all nuclear reactors using uranium.
It is possible to build and operate a special breeder reactor, whose purpose is to produce high quality plutonium-239 as well as, or even instead of, electrical power; this can then be used to make nuclear weapons or in a plutonium nuclear reactor. But the reactor must be shut down at quite short intervals in order to remove the fuel rods and recover the plutonium-239. In a (uranium) nuclear reactor designed only to produce power the reactor is not shut down so often, and the layout of the fuel rods in the reactor is such that the plutonium-239 becomes so contaminated with other substances, including other non-fissile isotopes of plutonium, that it is very difficult to separate it.
The plutonium-239, and all the other fission and decay products, build up inside the fuel rods, but are trapped in fixed positions within the lattice of uranium atoms. The other materials in the core are also being bombarded by neutrons and some of these may also become radioactive. When the reactor is completely shut down nuclear fission stops but radioactive decay does not: the heat produced by the decay of the radionuclides in the core and fuel rods is called after-heat. Although it is less than the heat produced by fission it must still be removed: in the event of the failure of the cooling systems the control rods should shut down the reactor, but if the after-heat is high enough the temperature may still rise high enough to cause damage to the zirconium casing of the fuel rods and allow the uranium fuel pellets to melt, so allowing the very highly radioactive fission and decay products to escape from their position in the lattice. This is called meltdown and is the worst-case nuclear reactor accident, as it may lead to the release of massive amounts of radioactivity. A partial meltdown occurred at Three Mile Island Nuclear Power Station on March 28th 1979 when the cooling system failed but the reactor did not shut down - you can read about it by going to this Web Site
The Three Mile Island incident did not lead to any release of radioactivity into the atmosphere; the Chernobyl disaster of 25th April 1986 led to the release of massive amounts of radioactivity. You can read about this by visiting one of the Chernobyl Web Sites
It is important therefore that the fuel rods are replaced before they are so radioactive that the after-heat is enough to allow this to happen; there are also many other safety and operational reasons why they must be changed a long time before all the uranium-235 has been used up. In the United States and some other countries with nuclear power stations the used fuel rods are just stored in deep underground bunkers; in Britain the used fuel rods are reprocessed - they are dismantled and the uranium is separated, enriched and re-used, leaving behind the depleted uranium, plutonium and other highly radioactive materials. This reprocessing is carried out at Sellafield; Sellafield also reprocesses fuel rods from some other countries with nuclear power stations such as Japan.
Many people believe that transporting very highly radioactive waste half-way across the World, or even half-way across Britain, is not the Wisest Thing we can do, and that nuclear fuel reprocessing is both unnecessary and dangerous: most radioactive leaks have happened at nuclear reprocessing plants rather than nuclear power stations. Additionally, the plutonium-239 in spent fuel rods is so heavily contaminated with other substances that it cannot be used for making nuclear weapons; it is only after the rods have been reprocessed that the plutonium-239 is in a form of any value those wanting to make nuclear weapons, such as terrorists and dictators.Existing stocks of weapons-grade plutonium-239 must be stored safely for thousands of years, but a much more efficient way of disposing of them is to use them to seed thorium reactors - this is discussed in the next section.

Thorium-232 is the most common and most stable naturally occurring isotope of thorium. It has a half-life of 14 × 109 years. When it is bombarded with neutrons it gains a neutron to become thorium-233. This is radioactive, with a half-life of 22 minutes. The thorium-233 undergoes a beta decay to form uranium-233. Uranium-233 is radioactive, with a half-life of 162 000 years, but it is also fissile, and its fission releases enough neutrons to sustain both the fission and the conversion of thorium-232 into thorium-233 and so uranium-233. Under the right conditions therefore a chain reaction can occur.
India is the world leader in thorium reactor development because she has about 13% of the world’s thorium deposits but almost no uranium, and as a country which possesses nuclear weapons but has not signed the nuclear non-proliferation treaty there are restrictions upon her ability to import uranium.
Experimental thorium reactors have been built in several countries, and India is now building fully commercial thorium reactor power stations. These have several advantages over uranium power stations: among others, they do not produce plutonium-239, and thorium is much more common in the Earth's crust than uranium.
Present-day thorium reactors need to be seeded with a highly enriched fissile material, for example plutonium-239, to build up enough thorium-233 to start the chain reaction. This fissile material is quickly used up, and then the reactor produces only just enough fissile material to sustain the reaction; the spent fuel rods contain no fissile material. A thorium reactor is therefore a highly effective way of using plutonium-239 - they have even been called plutonium incinerators.You can read more about this by going to a Thorium Reactor Web Site.
