- Methanol CH3OH
- Ethanol C2H5OH
- Propanol C3H7OH
- Butanol C4H9OH
- Pentanol C5H11OH
- Etc
The lower alcohols, with a few carbon atoms, are flammable liquids, and the higher ones, with many carbon atoms, are waxy solids. Cholesterol is one of the higher alcohols.
Organic chemistry is considered in greater depth on the Organic Chemistry Page.
Alcoholic drinks
“Alcoholic drinks” contain ethanol - when we talk about alcohol rather than an alcohol we almost always mean ethanol. Alcoholic drinks are made by fermenting natural sugar solutions.
- Mead from honey
- Wine from grapes
- Cider from apples
- Perry from pears
- Beer from malt
By international agreement, the word wine by itself may be used only to describe wine made from grapes. It is possible to make alcoholic drinks from many different plants but these must be called, for example, dandelion wine or, collectively, country wines. Many country wines are home-made or made only for local use and on a small scale.
Fermentation is carried out by microscopic fungi called yeasts. The yeast grows by turning sugar into ethanol and carbon dioxide. However, ethanol is a poison for yeast and once the concentration of ethanol reaches 10% to 15% (depending on the strain of yeast) the ethanol will kill the yeast and no further fermentation can occur, no matter how much more yeast or sugar we add. Alcoholic drinks stronger than this cannot be made by fermentation alone. Fermentation is further discussed in the page on respiration - to read this page please click ![]() .
.
The boiling point of ethanol is about 78oC, compared to 100oC for water, so if any alcoholic drink is left in an unsealed container the ethanol will evaporate at a higher rate than the water, and the alcoholic strength of the solution will fall - the higher the temperature the more rapid and the greater the fall.
Mulled wine is a very warming drink in cold weather. It is made by adding spices to heated red wine. If the wine is not drunk straight away most of the ethanol will evaporate and what is left tastes very different: it is as though the life has gone out of it. The Ancient Greeks used the word spirit to describe what was lost from the wine when it was heated. Later people discovered how to capture the spirit (that is, the ethanol) by distillation (evaporation followed by condensation) and today alcoholic drinks made by distillation are called spirits. Common spirits are
- Brandy, made from grapes
- Whisky, made from malt
- Gin, made from wheat (but flavoured with juniper berries)
- Rum, made from molasses
Spirits usually have an ethanol content of from 25% to 60%.
By analogy many other substances made by distillation are called spirits.
Apart from being present in alcoholic drinks, ethanol has very many other important uses, as a fuel and as a solvent, for preparing skin before injections, for cleaning and degreasing electrical equipment, and in many other ways. It is impossible to make ethanol stronger than 95% by distillation alone. The last 5% of the water can only be be removed by chemical processes, and as 100% ethanol (“absolute alcohol”) absorbs water very rapidly it is used only for the most specialised purposes. Ordinary “pure” alcohol is 95% ethanol and 5% water.
Fortified wines, such as madeira, sherry and port, are made by fermenting grape juice and then at exactly the right point adding exactly the right amount of brandy. This raises the ethanol content of the mixture to a level at which the yeast is killed, and fermentation stops. The fortified wine is then usually matured for several years.
The timing of the addition of the brandy, and the amount added, have a critical effect on the quality of the end product and require very careful judgement.
Fortified wines usually have an ethanol content of about 20% to 25%.
Sparkling wines such as champagne are made by carrying out the fermentation in two stages. The first stage takes place in open barrels or casks so that the carbon dioxide can escape, then the half-fermented juice is put into sealed bottles for the second fermentation. The carbon dioxide produced by this cannot escape so the pressure builds up and the carbon dioxide dissolves in the wine under pressure.
The fermentation must be carried out in two stages: if it were carried out in sealed bottles from the start the pressure build-up would be much greater and would burst the bottles - this is how Dom Perignon discovered how to make champagne!
The way champagne is made is actually quite complicated but you can read about it on this (American) web page. This method is very expensive to carry out; less expensive sparkling wines are made in less expensive ways, perhaps even just by carbonating wines made the ordinary way. (But they do not taste the same!)
Ethanol is absorbed into the bloodstream very rapidly, not only through the intestine and stomach but also through the lungs and nasal passages - thus drinking alcoholic drinks through a straw, where the fumes are inhaled, greatly increases the rate of absorption. Ethanol affects the central nervous system, and although different people are affected in different ways, for all people even small amounts of ethanol in the bloodstream can affect reaction times and judgement to the point at which driving and many other activities become unwise or dangerous. It also often leads to violent or anti-social behaviour. In larger quantities it causes drunkenness and eventually death.
Ethanol in the bloodstream is broken down by the liver. Small quantities, for example a glass of wine with a meal once a week, are unlikely to be harmful to healthy adults, but larger quantities, whether taken at one time or over a longer period, may cause long-term liver and brain damage and other health problems.
Genuine alcoholic drinks are always made by fermentation, but it is also possible to make ethanol from petroleum (oil), and fake wines and spirits are sometimes made with ethanol produced in this way. It is however very easy to detect these fakes because genuine drinks are made from plants which were growing quite recently and so contain carbon-14 and can be carbon-dated, whereas the original organic material in petroleum is millions of years old and so has lost all its carbon-14. Radio-carbon dating is described on another Page.
Vinegar
Many micro-organisms other than yeasts can also cause chemical changes to grape juice, and one of them, a bacterium carried by fruit flies, can cause a chemical change leading to the production not of ethanol but of ethanoic acid. (Ethanoic acid used to be called acetic acid.) Grape juice that has been affected in this way tastes sour and is called vinegar (from the French for sour wine). For obvious reasons, in wine-making countries fruit flies are more commonly called vinegar flies.
Vinegar can also be made from malt and cider etc. In Britain most vinegar is made from malt and is brown.
Methanol and methylating
Methanol is very poisonous and causes blindness and death. It can be made by distilling wood, hence its common name wood spirit.
In Britain and most other countries there is a tax on alcoholic drinks based upon their ethanol content, and pure ethanol has such a high rate of duty that it would be far too expensive to use as a fuel or for other purposes. To avoid the tax it is mixed with a small amount (about 5%) of methanol to make it poisonous. This is called methylating it, and because this mixture is poisonous it is not taxed. A mixture of ethanol and methanol is called industrial methylated spirits, or IMS. IMS is a clear colourless liquid. Methanol and ethanol are very similar and for almost all purposes (other than drinking of course!) IMS can be used instead of ethanol. In school science laboratories bottles labelled “ethanol” are almost invariably IMS.
It is however possible (if you know how) to separate the methanol from the ethanol in IMS and for this reason in England you cannot buy IMS without a special permit from the Customs and Excise - most secondary schools have such a permit. Mineralized methylated spirits (ordinary meths) is a mixture of ethanol and methanol, with the addition of a purple dye to make it easily recognizable, and another substance to give it a revolting taste and to make it more difficult to separate the ethanol from the mixture. This can be bought by anyone over sixteen. These extra substances do not prevent meths from being used as a fuel but do make it unsuitable for many other purposes including use as a solvent in school science experiments.
Even though meths tastes revolting and is very poisonous it is more than 90% ethanol and some alcohol-dependent people on the very edges of society do drink it - meths drinkers usually go blind within a few months and die soon afterwards.
Surgical spirit is used by doctors and nurses to prepare the skin before injections, and also as a rub to harden the skin to prevent bed sores. It can be bought at pharmacies. It is about 90% ethanol and 5% methanol with a small amount of methyl salisilicate (“oil of wintergreen”) which acts as a mild painkiller.
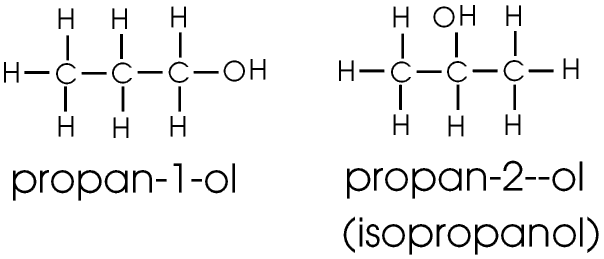
Propanol (for Advanced Readers)
Propanol and all alcohols with three or more carbon atoms exist in the form of two or more isomers, substances with the same chemical formula but slightly different arrangements of atoms. Isomers are discussed on the Page on Organic Chemistry.
Propanol does not affect us in the way that ethanol does so we do not drink it, and it is not taxed. It exists as two structural isomers, propan-1-ol and propan-2-ol or isopropanol.