
This Page was originally only on lighting in Ancient Egypt but is being expanded to cover lighting up to the introduction of electriity.
If you are reading this Page on your computer screen or tablet you probably live in a town or village where you take electric lighting for granted: if it gets dark indoors you turn on the light; if you go out at night there are street lights everywhere. But until less than a hundred years ago it was not like this at all: even today in many European countries there are still whole villages where there is no mains electricity; in Africa and Asia there are still whole countries where there is no mains electricity except in the very largest towns. Without electricity almost the only way you can make a light is by burning something.
The main sources of lighting in Ancient Egypt, and the rest of the world before the Industrial Revolution, were oil lamps, candles and torches - the word torch has taken on a new meaning since about 1900. The Sections on these were originally written for Junior School children, but I know from my e-mails that other people find them interesting.
The paraffin lamp, the gas lamp and the miners’ safety lamp have all been introduced since about 1800 CE, and the Sections on these are necessarily slightly more technical and are written for Secondary School children.

Oil lamps burn a liquid. Many different liquids can be used, and we usually refer to any liquid used in a lamp as a lamp oil. The oil lamps you can buy in the shops today usually burn paraffin. Paraffin is made from petroleum from oil wells, but the first oil well was not drilled until 1859, so until then almost all lamps burnt oil extracted from animals or plants - in many parts of the world they still do.
Paraffin lamps are quite different from lamps burning oils from animals or plants, and are discussed in their own Section.
You can make lamp oil from many different plants by squeezing the oil out of their nuts or seeds. Today the cooking oil you can buy in your local supermarket is often a blend (mixture) of several different vegetable oils, some from plants which do not grow in Britain. But the Ancient Egyptians and other ancient people could only use plants which grew near where they lived. The Greeks and Romans used olive oil, and still do, but olive trees did not grow in Ancient Egypt, so the Ancient Egyptians used oil from flax, walnuts and almonds and other nuts, sunflower and sesame seeds, wheat and castor oil plants. The finest lamp oil, used in Pharaoh’s Palaces and in the Temples, was made from sesame seeds.You can also make a lamp oil from soft animal fat, but this produces a lot of very unpleasant smoke when it is burnt. This is the cheapest lamp oil. The Ancient Egyptians used this when they were digging out the Pharaohs’ tombs in the Valley of the Kings, but not when they were doing the paintings on the walls inside the tombs as the smoke would have spoilt them. We know this because the Egyptians kept written records of everything, including the amount and type of oil used every day by the tomb builders!
You can also make lamp oil from the livers and other parts of sharks and certain other “oily fish” which live in the sea, and from whale and seal blubber. Inuit and many other people still make lamp oil this way, but of course the Ancient Egyptians did not, because they only caught fresh water fish in the River Nile.
In high mountains such as the Himalayas and Andes no plants grow which humans can eat, so the people who live there are almost totally dependent on animals such as yaks and llamas and goats which can eat the plants that can grow there. Milk, whether from yaks or llamas or goats or any other mammals, can be drunk straight away, before it goes sour (without refrigerators and pasteurisation in only a few hours) or turned into butter or cheese or yoghurt, and butter can be used in lamps - even today the Temples in Tibet are lit by lamps burning butter. Although the Ancient Egyptians milked cows, sheep and goats they had plenty of other sources of oil for lamps so did not use butter.
Although soft animal fats and butter are solids at room temperature they are usually too soft to be made into candles so they can only be used in lamps: the fat or butter is spooned rather than poured into the lamp, but once the lamp is lit the heat melts it so the lamp is filled with liquid.
It is possible to treat certain types of animal fat to make them into a substance called tallow which is much harder and which can be used for making candles; this is described further in the Section on Candles.
Most substances, including of course the oil in a lamp, will not start to burn unless you heat them first.The ignition temperature of any substance is the temperature we must heat it to before it will start to burn. The combustion temperature of a substance is the temperature which is reached as it is burning, this will depend upon a number of factors, including for example how much air is available (this is discussed more fully in the Section below, on the Yellow Flame), but will always be higher than the ignition temperature. Once a small amount of the substance has been raised to its ignition temperature and started to burn the heat produced may raise some more of the substance to its ignition temperature, and so that will start to burn. If you were to put a match to a chip pan full of cold cooking oil (but please don’t!) the match would go out and the oil would not catch fire: the heat from just one match is not enough to raise any of the cooking oil in the pan to its ignition temperature. But the ignition temperature of cooking oil is only slightly higher than the temperature needed to cook chips, and so if we are cooking chips by heating a whole pan of cooking oil on a hob and take our eyes off it for even a few seconds the oil may overheat and reach its ignition temperature and catch fire. It will then burn very quickly with clouds of thick black smoke. A chip pan fire is very dangerous - and would be no use at all for lighting a room! If we want to burn any sort of oil to light a room we must use a lamp with a wick.
Take a strip of newspaper or kitchen roll and dip one end into a bowl of water. The water will start to rise up the paper. You can see this more clearly if you add a few drops of ink or food colouring to the water. The paper acts as a wick. Most liquids (except mercury) will rise up a wick in this way; how far they rise up the wick depends upon what the liquid is and what the wick is made of.
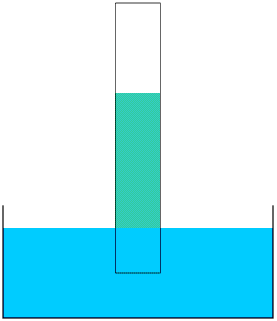
Newspaper is fine if you just want to show how a wick works, but a paper wick is useless for anything else as it will just fall to pieces as soon as it gets wet: the best materials for wicks are natural fibres such as flax and linen, wool and cotton. The Ancient Egyptians made their wicks by twisting together the fibres from flax plants. Here is the hieroglyph for a twisted flax wick: the Egyptians also used this hieroglyph for a h sound.
A lamp consists of a container for the oil with a wick dipping into the oil. The oil rises up the wick. If we then put a match to the end of the wick the heat of the match is enough to raise the small amount of the oil on the wick to its ignition temperature and it will start to burn with a yellow flame, giving out light. As the oil is burnt up fresh oil rises up the wick to take its place and so the lamp will continue to burn and give out light. Why the flame is yellow is described in the slightly more technical section Why is the flame yellow?
The ignition temperature of the wick is much higher than the ignition temperature of the oil, so as long as all of the wick is wet with oil it will not catch fire, although if it dries out in places it may char (go black and crumbly, like charcoal). Then the wick must be trimmed using special scissors. You must not allow the lamp to burn dry (run out of oil).
A lamp will not work without a wick, but it is the oil that is burning, not the wick.Paraffin rises up a wick quite a long way, but most animal or plant oils only rise up a wick a few millimetres - this is why paraffin lamps are different from those burning animal or plant oils. Lamps burning plant or animal oils must have very short wicks above the surface of the oil. The oil in the container does not catch fire because the heat from the flame is not enough to raise it to its ignition temperature, only the small amount of oil on the wick above the surface reaches this temperature.
Greek and Roman lamps were usually shallow dishes made of gold, silver or bronze or stone or pottery, with a cover to stop the oil from spilling and a small hole at each end, one for the wick and the other to fill it with oil. Here is a photograph of a modern reproduction of an Ancient Greek lamp. Aladdin’s magic lamp was like this only made of gold.

Lamps like this, burning vegetable or animal oil, have to be quite wide and shallow to hold enough oil because the part of the wick above the surface of the oil has to be very short. If the lamp were tall and thin, once it had been burning for a short time the oil level in the lamp would have gone down too much for it to rise up the wick far enough to burn.
It is not usually possible to adjust the length of the wick, and so the size of the flame, while the lamp is burning (unlike paraffin lamps).Lamps like this can be small enough to be carried around in your hand although you need to be careful not to tip them. Larger lamps would always be put on a lampstand or in a sconce: a sconce was a special wall shelf or wall recess or wall bracket where lamps (or candles or torches) could safely be left while alight. A lighted lamp, even one normally carried in the hand, would never be put down anywhere than on a lampstand or in a sconce, and a sconce was never used for any other purpose: safety with lighted lamps was paramount. Sadly today we are so used to electric lighting that we are often very careless with lamps and candles, particularly during a power cut...
Lamps and candles are lit and extinguished (put out) in almost exactly the same way, but because today most people use candles rather than lamps the processes are described in the candles sectionEgyptian lamps often used a floating wick. The wick was threaded through a metal or ceramic ring which was mounted on a float of some sort. This meant that the container for the oil could be any size and shape.

Most of us have seen, even blown out, candles on a birthday cake. Birthday cake candles work in exactly the same way as all other candles, but we use them in such a different way that they are not considered in this Section except at the very end of it.
A candle uses a wick just like a lamp, except that at room temperature the fuel is a solid wax. The wick runs down inside the candle for its full length, but of course you can only see the tip of it. The heat of the flame melts a small amount of the wax and the liquid rises up the wick and burns in the flame - remember it is the molten wax that is burning not the wick.
Many different materials have been used for making candles: tallow candles were made of animal fat rendered in a special way so that it was much harder and did not putrify (go bad, like meat would) and was not eaten by maggots or other animals. Tallow candles were the cheapest but made a lot of unpleasant-smelling smoke. Today most candles are made of paraffin wax, but other substances including beeswax can also be used, and candles are often scented.
As the candle burns it gets shorter. The wax only rises up the wick a few millimetres, so the tip of the wick will soon not be wet with wax. It is designed to burn away as this happens, so keeping the wick the right length. Sometimes the wick may not burn away correctly so it will get too long and go floppy, and then it must be trimmed with special scissors.
The size of the candle and the thickness of the wick must be matched very carefully so that the puddle of molten wax produced by the flame is exactly the right size. If it is too big molten wax will run down the side of the candle and be wasted, and the candle will not last as long as it should, but if it is too small the edge of the candle will not melt and the flame will eventually be hidden by it and it may go out. In either case, after the candle has been extinguished and the wax allowed to cool the wasted wax must be cut away with a knife to restore the candle to the correct shape for it to be relit.
If the candle is in a draught both may happen at the same time, with wax pouring down one side. A candle in a draught can disappear into a pool of wax in a few minutes, this is called guttering.
A candle to be used out of doors or in a large building where draughts are likely is usually put inside a glass tube to protect it from the wind. Candles are often used inside churches or carried in processions like this.
A lighted candle can also be carried inside a metal box with glass sides, or often just one side: this is a lantern. A dark lantern carried by a criminal, and also Mr Sherlock Holmes, has a cover that you can place over the glass when you need to, to stop anyone from seeing the light.
Any candle much longer than it is wide should always be put into a candlestick or other candle holder before it is lit to stop it from falling over: it is tempting if you are using a candle during a power cut to melt the wax on the bottom and use this to stick the candle onto a saucer, but you should never leave a candle like this in a room where there are children or elderly people around, or in an empty room.
Ornamental candles which are much thicker do not need to be put on a candlestick, but they must be put on a flat heat-resistant surface, and of course must never be left unattended.

You should never ever carry any sort of lighted candle in your bare hands without some sort of wax shield: if hot wax runs down onto your hands you may burn yourself or drop the candle and set fire to the house, or both.
Lighting and putting out a candle looks easy (like laying bricks) but you do need to know what you are doing. If you just hold a flame to the wick you will only char the wick, so making it less effective when you eventually do get the candle to light. Unless you hold the flame to the candle in a way which allows it to melt some wax the candle will not light. Do not blow the lighter out until you are certain that the candle is properly alight, and then (promise not to laugh) make certain you do not blow the candle out at the same time - I thought you promised not to laugh! - it really does happen.
Today you can light just one ordinary household candle at waist height with a match, but if you want to light several candles, or a large candle, or a candle with its top above shoulder height it is best to use a taper, and until matches and gas lighters were invented this was the way all candles were lit.Candles in churches are almost always lit with a taper.

A taper is like a long thin candle. Today they are usually made of paraffin wax, but in the past many different substances were used, they could even be just a hollow reed dipped in melted animal fat.
Tapers more than about 30 cm long are usually contained inside a metal tube which they can be slid through, leaving just the tip exposed, so that they do not bend under their own weight. The tube can then be fixed to the top of a pole.
A gas lighter is so-called because it is fuelled by a gas such as butane, although you can of course use one to light a gas hob or oven as well as a lamp or candle - more about this on the Storage of Gases Page. A cigarette lighter is usually the wrong shape to light a candle.

You can blow out an ordinary domestic candle below mouth level quite easily, but again you must know what you are doing: if you do not do it right the wick may go on smouldering for a time, and you may blow the molten wax into a shape which when it solidifies may make the candle difficult to light next time. It is always better to use a snuffer, and of course you must use a snuffer if the candle is above mouth level. A snuffer is often put on the same pole as a taper.
Candles were once used as simple clocks or timers: you marked out the hours on the side and watched them as the candle burnt down. You could also use a candle to time something, not in minutes but in inches of candle. A pin was stuck into the candle an inch (25 mm) from the top - the time was up when the pin fell out. You could not hear the pin fall if you were making a lot of noise, hence the expression “quiet enough to hear a pin drop.”
Candles have many disadvantages compared with oil (or paraffin) lamps, and candle wax is more expensive than lamp oil: their advantage over lamps is that unlit candles are much easier to carry around and store than containers of oil, and of course you do not need to own a lamp.
Some of the disadvantages of candles, particularly guttering, can be overcome by using cased lights, where the wax is contained inside a metal or glass or plastic case. Cased lights can be of a size to burn for just an hour, or several hours, but a cased light burning in front of a statue in a church can burn for a week.



Glass cases can be reused, metal and plastic cases can be recycled.
Cased lights are often scented or treated in special ways, for example to make “smokers’ candles” to get rid of the smell of tobacco smoke.
Birthday cake candles are ordinary candles, except that cost is not a factor, they burn for only a very short time, they are not usually relit after they have been used once, guttering or uneven burning is not a problem, and they are never left unattended. And of course there is no reason to use a candle snuffer, blowing them out is perfectly OK.
If you ever make a wish when you blow out a candle, or drop a coin into a Wishing Well, or at any other time, and ever have a spare wish left over, could you wish something, not for me but for the young people I teach and who use my Web Pages: that they could be half as good at remembering the things I tell them that are important as the things I tell them that are not.

Originally Man used fire to cook his food, keep himself warm, and frighten away wild animals, and also to sit round after sunset and tell stories and sing - most books do not mention this use of fire. Story telling and singing was an important part of early Man’s life, and in many parts of the world it still is.
Then more than thirty thousand years ago Man began to use torches: a torch is just a small fire on the end of a pole which can be carried around. The pole must be longer than you are tall and held upright so you do not burn yourself or anyone near you and the light from the fire does not shine in your eyes.
The word torch has taken on another meaning over the past hundred years, but lots of people, mainly children and young people, still know about the old sort of torch because they use them in Minecraft.
What the fire was made of and how it was fastened to the pole depended upon what materials were available: today in Minecraft they are sticks with lumps of coal or charcoal placed on the top, but in the world outside Minecraft this would not work very well: anyone who has ever had a barbeque knows that burning charcoal does not give out much light, and coal is very little better.
In Scandinavia and Germany and Scotland there are lots of pine trees and other trees containing resins which burn very well, so their torches would have been made of thin branches of these trees tied together in bundles with leather straps. Today in films Viking warriors are shown carrying torches like these.
In Southern England and France the wood from the trees which grow there does not burn so freely so they used splinters, logs split lengthways into very long thin pieces (like today’s splints) so that they burnt better (see the Section on Why is an oil lamp flame yellow?), in other places they used knotted ropes soaked in oil or covered with animal fat. Absolutely anything that would burn and could be fixed onto the end of a pole could be used.
We know that torches were being used thirty thousand years ago because we can date the wonderful paintings in caves to this time. Some caves have paintings in places that must have been very difficult to reach, other caves have no paintings at all. The reason is the acoustics of the cave. Some places are lovely to sing in, others are quite “dead” and we think that the artists were singing while they were painting!
Torches could only be used in the open air or in caves, not in tents or shelters. Thousands of years later, when Man was living in cities, with houses and palaces and temples, torches could be used in large stone or brick buildings with high ceilings, such as the Great Hall of a King’s Palace, but not in a peasant’s cottage with a thatched roof!
Once Man learnt to use metals he could put the fire into a metal basket, a fire basket. This made the torch much safer and easier to use. A fire basket could also be used as a beacon, a fire in a basket on a pole in a very high place where it could be seen from a long way away. Beacons like this could be used to guide travellers or lit to send warnings, for example that the Spanish Armada had been sighted. Lots of places still have beacon in their name, for example Dunkery Beacon on Exmoor is more than 500 m above sea level and when the beacon on the top is lit it can be seen from more than 100 km away.
A torch was lit from a fire or another torch; when you did not need it any more you let it burn itself out, in a safe place of course, or poured water on it.
Today torches like this are only used in historical re-enactments and in some religious processions, and in common speech we use torch to mean a small hand-held battery operated electric light. But beacons are still used today to celebrate special occasions, for example the Queen lit a beacon on her 90th birthday and this started the lighting of more than a thousand more all over the country.

Most modern oil lamps burn paraffin, a mineral oil made from petroleum. This rises up a wick several centimetres, so paraffin lamps can be almost any shape and size, although the wick is usually flat rather than round - you can see this in the picture. Often the paraffin is coloured or scented.

The top of the wick runs through a metal collar, and a knob at the side allows the height of the wick above the collar to be adjusted to make the flame bigger or smaller, even while the lamp is alight. By the time paraffin lamps were first used matches had been invented so a paraffin lamp is usually lit with a match; you put out a paraffin lamp by winding the wick down inside the collar, but of course you need to be careful not to wind it down too far.
The collar must be metal: this conducts the heat of the flame away so the the part of the oil-soaked wick below the collar, which is exposed to the air, never reaches the ignition temperature of the oil. This is more fully discussed in the Section on The miners’ safety lamp.
Paraffin lamps are still used for many purposes today: I use them during a power cut - much better and far safer than candles. A hand-carried paraffin lamp designed for use out of doors in strong winds is often called a hurricane lamp. Paraffin lamps can be tilted a small amount quite safely without going out, although on ships and boats they are usually mounted on a gimbal so they stay almost vertical when the vessel pitches or rolls. The children in the Swallows and Amazon Books used paraffin lamps on their boats although they used electric torches in their tents.
Paraffin heaters are described in the section on the Tilley Lamp. A Tilley lamp burns paraffin but in a quite different way, it does not use a wick.

This Section is here mainly to help you understand the Section on gas lighting, and does get a little technical. Gas lighting was not introduced until after 1800 CE so if you only want to find out about lighting in the Ancient World you have already read everything you need.
All fuels except hydrogen contain carbon. Hydrogen is used to power space rockets, and it can also be used as a fuel in lots of other ways. When it burns it produces nothing but water, and it can be made from water using electricity. So if we use electricity from carbon-free sources such as wind farms or nuclear power stations to make it, hydrogen is the perfect fuel for combatting global warming, and there are lots of very exciting things happening in this field. But hydrogen is not used for lighting so it is not considered further on this Page.
Coal is impure carbon. It is not used for lighting although coal gas, made from coal, used to be, and this is discussed later under Gas Lights. All other fuels (except charcoal, which is discussed later in this Section) contain not particles of solid carbon but solid or liquid or gaseous compounds of carbon and hydrogen, and also often other elements.
Fuels need oxygen, present in the air, to burn. If we burn a fuel in lots of air the hydrogen burns to form water (water vapour of course) and the carbon burns to form carbon dioxide. If however there is not enough air to burn both the hydrogen and the carbon the hydrogen will take precedence over the carbon, and after it has burned there may not be enough oxygen left to completely burn all the carbon: some may burn to form carbon monoxide, and some may not burn at all, but will be left as tiny particles of pure carbon (soot). These soot particles are heated by the burning hydrogen, and the yellow flame is caused by them glowing in the heat!
The soot from a lamp is very pure carbon. It used to be called lampblack and was used for all sorts of purposes. The soot from coal and wood fires is also carbon, produced in exactly the same way, but it also contains other substances so was less useful than lampblack. The soot from a coal or wood fire collected in the chimney and had to be removed at regular intervals so that it did not build up and eventually catch fire: in Victorian England young boys were sent to climb up inside the chimneys to sweep them.
When you go swimming you do not all get wet, only your outside (skin). Similarly if we have a cube of wood only the outside of the cube is exposed to the air. If the cube has a side of 2 cm its volume is 2 cm × 2 cm × 2 cm or 8 cm3, and its total surface area is 6 × 2 cm × 2 cm or 24 cm2. If however we cut the cube up to make 8 cubes each with a side of 1 cm we keep the volume the same but the surface area is now 48 cm3, so a much larger area is exposed to the air.
If we have a large lump of wood on a fire it will burn slowly and may not burn completely, so some of the carbon may be left, in the form of charred wood, but if we poke it with a poker we can break it into smaller pieces and this increases the surface area and so allows it to burn more quickly and more completely. (Charcoal is discussed later.)
Similarly with a liquid such as a lamp oil, except that a liquid must be in a container, so only a small part of the surface is exposed to the air. Even if you spill it on the ground only half of its surface is exposed to the air. A wick gives the small amount of the oil in the wick a large surface area. If the surface of the oil-soaked wick exposed to the air is not big enough to allow all the oil to burn completely we shall get a yellow flame, as the tiny particles of unburnt carbon (soot) glow in the heat of the burning hydrogen. This is of course what we want in a lamp. Such a lamp will produce, as well as soot, lots of water vapour, some carbon dioxide and some carbon monoxide. Carbon monoxide is a poisonous gas but the amount produced by a single oil lamp in a well-ventilated room would not usually be likely to be dangerous.
Paraffin heaters are discussed in the Section on the Tilley Lamp.
Wood is made up of compounds of carbon and hydrogen, phosphorus, potassium, nitrogen, sulphur and lots of other elements. When we burn it completely in lots of air the carbon, hydrogen and sulphur burn to form gases, and the phosphorus and potassium and other elements burn to form solids - these solids become the ash. If we burn it in plenty of air we end up with just a small pile of ash, but as we reduce the amount of air the wood begins to char rather than burn, and if we go on reducing the amount of air we end up with charcoal, almost pure carbon, but the charcoal retains the shape of the wood, and is very porous. Charcoal has many uses, one of which is of course as a fuel in barbeques, because it burns easily with no flame or smoke, but it does need the air to be circulating through it.
Graphite is another form of almost pure carbon. It occurs naturally in rocks in the Earth’s crust and has many uses. It is used in “lead” pencils and in carbon fibre, for example fishing rods, but it cannot be used as a fuel or in the extraction of metals (see below).
Soot and graphite do not usually allow the air to circulate through them so it is not easy to set fire to them, but if they do start to burn the carbon dioxide produced puffs them up, like bread rising, and then they burn in a way very difficult to put out. A chimney fire, where the soot catches fire, is very dangerous; what changed what happened at the Chernobyl nuclear power station in April 1986 from a local accident into an international catastrophe was that the graphite core of the reactor caught fire and burned uncontrollably for nine days, releasing huge amounts of radioactive material into the atmosphere.
We need carbon to extract metals such as copper and tin and lead and iron from their ores, (this is discussed on another Page) but it must be very pure carbon, we cannot use wood or coal because of the other elements, particularly phosphorus, in them. For more than three thousand years charcoal was used, but then early in the 18th century CE it was discovered how to make iron using coke, a different form of almost pure carbon. Coke is made from coal, and making iron from coke rather than charcoal brought down its cost - this was the beginning of the Industrial Revolution. Lots of coke (and so coal) was needed to make all the iron, but coke-making also produced coal gas as a by-product, and soon coal gas was being used for lighting.
Coal mining is discussed in the Section on The Miners’ Safety Lamp.

Today the gas that is piped into our homes comes from deep underground. We call it natural gas, and it is mainly methane, with a harmless smelly gas added to it so we know if we have left the gas on or there is a leak. But until the 1960s (in Britain) the gas that was piped into our homes was coal gas, made from coal. Coal gas was a mixture of several different gases including carbon monoxide which is poisonous. There is more about natural gas and coal gas on another Page.
Initially coal gas was used only for lighting: it was not mixed with air so burnt with a yellow flame, as for an oil or paraffin lamp. Pall Mall, a street in London, was lit by gas in this way in 1807, the World’s first street lighting. Then in 1857, fifty years later, a German scientist called Robert Bunsen (1811 - 1899) realised that if you mixed the gas with air before you burnt it you could burn all the carbon as well as all the hydrogen, so the flame would give out much more heat and be much cleaner, and you could use it for heating purposes. He passed his ideas to his assistant Peter Desaga, who made the first Bunsen burner. This admitted air to the gas through an adjustable hole so that you could vary the temperature of the flame. (Turning the gas up or down adjusts the size of the flame but not its temperature.)
With the airhole shut the flame is yellow and very easy to see, as you open the the air hole the flame gets hotter but less easy to see, and eventually it is very hot, pale blue, and very difficult to see. You should never heat anything with the yellow flame (unless you want to come in at break to wash the soot off everything!) but for safety reasons in a laboratory or workshop you should always shut the airhole when you are not actually using it so that the flame is clearly visible.
A gas oven or hob or fire or water heater works in exactly the same way except that the airhole is concealed inside the equipment and is not adjustable.
If you heat a piece of limestone (calcium carbonate) in a very hot flame it decomposes to form lime (calcium oxide) and if you heat lime in a very hot flame it gives off a brilliant white light. This was soon being used for lighting the stage in theatres, hence actors were said to be “in the limelight.” There is more about this on the Lime Cycle Page.
Blocks of lime react very violently with water so limelight was unsafe to be used in homes; the final stage in the introduction of gas lighting in homes was the gas mantle. Thorium oxide also gives off a brilliant white light when it is heated but is much safer than calcium oxide. A piece of linen (the mantle) is soaked in a suspension of thorium oxide in water, and then allowed to dry. Particles of thorium oxide are left on the linen. Mantles are quite safe to handle like this, and you can still buy them in the shops. You fix the mantle onto the gas lamp. Then you set fire to the linen (with a match, not a gas flame!) and it burns away leaving just the thorium oxide in the shape of the mantle. Now when you light the gas you get a brilliant white light. You can see the mantle on the photograph of the Tilley lamp.
Only one problem: once the linen has burnt away the mantle is very fragile so you must be very careful not to let anything touch it, and make certain you always have some spares.
Gas lights were usually lit by a tiny pilot light, as in a gas water heater today, this made it less likely that the maid would damage the mantle when turning them on or off.
Once the gas mantle had been developed most homes in towns were lit by gas until the coming of electricity.
As well as methane there are three other gases which we can get from oil and gas deposits: ethane, propane and butane: we call them the petroleum gases. We do not use ethane as a fuel but we can polymerise it to turn it into polythene (this is described on the Organic Chemistry Page). But propane and butane are widely used as fuels where there is no (natural) gas supply as they can be stored as a liquid under pressure. Propane must always be stored in strong steel cylinders, but butane can also be stored in small light-weight disposable cartridges, for example Camping Gaz®. The general term is LPG, for liquified petroleum gas. There is more about this on the Storage of Gases Page.
Today LPG is used in cars, medical centres in remote areas, ovens and heaters in boats and caravans and homes without a piped (natural) gas supply, in gas lighters, and of course in gas lamps in marquees and on camp sites.
Gas lighters (that is, lighters fueled by LPG) can be used for lighting candles, oil lamps, fires, barbeques and almost anything else, including of course gas ovens and hobs. The gas lighter itself is almost always lit by a piezoelectric spark. Cigarette lighters use gas as a fuel but they are usually of a shape which makes them unsuitable for lighting anything else.

The beginnings of the Industrial Revolution were in about 1710 when iron was first made using coke (made from coal) rather than charcoal (made from wood). Over the next two hundred and fifty years whole towns grew up alongside the mines needed to provide the coal. Coal is still being mined today in many parts of the World.
Coal mines are dangerous places, deep underground, without any natural light, the roof dripping with water, and often filled with explosive, smelly, poisonous or suffocating gases. Until the introduction of (battery operated) electric lighting from about 1900 they were very dangerous places; until the introduction of the miners’ safety lamp in 1815 they were very very dangerous places.
The different gases in a coal mine were called “damps” from the Old Dutch dampf for vapour, for example methane which is very inflammable was called firedamp, while hydrogen sulphide which has a foul smell was called stinkdamp. Using an ordinary lamp or candle in a tunnel full of firedamp is Not A Very Good Idea and there were often underground explosions. The explosion used up most of the oxygen in the air, leaving just carbon monoxide, which is poisonous, and carbon dioxide and nitrogen, which are suffocating, and a lot of coal dust. This mixture was called afterdamp. Many people who were not killed by the explosion of the firedamp died from the effects of the afterdamp.
In 1812 there was a great explosion in a coal mine near Newcastle and lots of men and boys were killed. As a result two very different people each designed a safety lamp, which could be used underground without setting off an explosion. Their designs were as different as the people who designed them.
Metals are good conductors of heat. If you sit on a cast iron seat in the local park in the middle of the winter wearing a thin pair of trousers you will soon have a very cold bottom. I am told you get the same effect if you are wearing a thin skirt but I have never tried it for myself. The metal conducts the heat away from your body.
In the same way we can use something metal to conduct the heat away from the flame inside a lamp, so that the firedamp in the air around it does not get hot enough to reach its ignition temperature. If you are at school you can ask your science teacher to demonstrate this with an iron gauze. (Cooks also sometimes use an iron gauze on a gas hob when preparing special dishes, but not every home has one.)
A gauze is made by weaving threads to make a very open material, like a gauze bandage. It is called gauze from Gaza, the place in where the technique was first used. Gauzes can be woven from natural fibres such as cotton or linen or silk (but not often wool) or synthetic fibres such as nylon or polyester, and can be used in many different ways, in clothing, in bandages, for separating juices from fruit pulp, as mosquito nets, and much more. Gauzes can also be made from many metals, and can be used in sieves and tea strainers, as fly screens, in the catalytic converter (cat) in the exhaust system of a petrol-engined car, and in many other ways, but, for reasons not explained here, if a gauze is to be heated it must almost always be made of iron.
In science laboratories you may sometimes use an open gauze, but when you are heating a liquid in a glass beaker you usually use a ceramic gauze, with a ceramic patch in the middle. For this experiment you need an open gauze.
Place the gauze on a tripod over a bunsen burner. Open the airhole. If you light the flame below the gauze the gauze conducts the heat away so quickly that the gas above it never reaches its ignition temperature; light the gas above the gauze and the gas below it will not light. You can show that there is unburnt gas above, or below, the gauze by lighting it.
Sir Humphry Davy (1778 - 1829) was a very famous scientist. The mine owners asked him to invent a safety lamp, and he came up with a lamp in which the flame was surrounded by a gauze made of iron wires. Air could pass through the gauze into the lamp, allowing the flame to burn. If any firedamp were present it would also pass into the lamp and burn, and this would change the colour of the flame, allowing the miners to know that firedamp was present. But the gauze would take away the heat, preventing the firedamp outside the gauze from reaching its ignition temperature. The lamp itself had to be made of brass, because if you hit anything made of iron against a stone you may get a spark, which would be dangerous down a coal mine - flints have been used for firelighting for thousands of years.
George (Geordie to those who knew him) Stephenson (1781 - 1848) was an engineer, and lived and worked in Newcastle and the other coal mining communities in the North of England, and he was well known and highly respected by them. He designed and built mining equipment and railways and locomotives to haul coal around the North of England, and went on to build the World’s first passenger railway. He knew far more about coal mining than Sir Humphry Davy and after the 1812 explosion he also invented a safety lamp, but his lamp did not use a gauze, it worked in a totally different way. (Hence of course it was so ridiculous that anyone could consider that he had stolen Sir Humphry Davy’s ideas - see below.)
If you have a mixture of methane and air and ignite it at one point the flame will spread out from this point at a certain rate. In a bunsen burner the mixture of methane and air is travelling up the tube faster than the rate at which the flame is travelling so the methane in the tube does not catch fire. If you start with the airhole fully open and the gas tap fully on, and then very slowly turn the gas down you may sometimes be able to reduce the speed of the flow enough to allow the flame to travel down inside the tube, and then for a very short time you may sometimes get a tiny yellow flame at the airhole.If you are using gas (LPG) from a cartridge or cylinder rather than mains gas the pressure is much higher and if you turn the gas fully on you may get a gap between the top of the burner and the flame, in extreme cases you may even blow the flame out!
Hot air rises, so a flame produces an upcurrent of air. If you hold your hand in the flame of an oil lamp you will burn yourself, but if you hold it a few centimetres above it the very hot gases will have mixed with cooler gases and you will not.
Stephenson’s lamp consisted of two parts. At the bottom of the lamp there were a number of narrow brass tubes. These restricted the air flow and so increased its velocity to above the speed at which the methane flame could spread. At the top of the lamp there was a long but wide copper tube which allowed the hot gases from the flame to cool to below the ignition temperature of the firedamp before they mixed with the air in the mine.
This picture shows just the workings of the lamp not the lamp itself which would have been made of brass, with a handle, and bars round the glass to protect it. But you can see pictures of all the different lamps by doing a web search on miners’ safety lamps. But be warned: some of these sites are very technical while others are far too simple to satisfy enquiring young minds.
The narrow tubes at the base of the lamp restricted the airflow to just enough to keep the lamp burning. If there was a very small amount of firedamp in the air it would enter the lamp and burn but not ignite the firedamp in the air outside the lamp, but more firedamp in the air would mean less oxygen in it and the lamp would go out.
This contrasted with the Davy lamp where there was no restriction on the air coming into the lamp. If firedamp was present this would burn inside the gauze and the more the firedamp the hotter the gauze would get: if it got red-hot it might ignite the firedamp outside the lamp and cause an explosion.
Initially most of the mine owners used the Davy lamp in their mines - after all they had asked Sir Humphry Davy to design it and he was a very famous scientist. Only the Newcastle miners, who did not know Sir Humphry Davy but who knew and trusted their Geordie, used the Geordie lamp - hence the Newcastle miners, and soon everyone else from Newcastle, were called Geordies.
Sir Humphry Davy was furious that someone else had made a safety lamp, and accused Stephenson of stealing his ideas: how could a mere engineer, with no science training, who could not even read until he was eighteen, and with such a dreadful accent, have invented such a lamp? Soon everyone else recognised the merits of the Geordie lamp, but Sir Humphry Davy continued his vitriolic campaign against Stevenson until the day of his death, and thus ensured that he is still given sole credit for its invention.
The Davy lamp did have two BIG disadvantages compared with the Geordie lamp. The gauze had to be made of iron, and it soon started to rust in the very damp conditions down a coal mine, and once this happened the lamp was no longer safe - several gas explosions in mines were caused by Davy lamps. And in the Davy lamp the flame was surrounded by a metal gauze, in the Geordie lamp it was surrounded by a glass.
Of course quite soon after the Davy and Geordie lamps had been invented other people were using the ideas upon which these two were based to make better ones. A web search on history of the miners’ safety lamp will give lots more information.