The way in which carbon atoms are reused again and again by living organisms is called the Carbon Cycle. All life on Earth depends upon the Carbon Cycle.
Many school text books and courses on the Carbon Cycle do not include the part played by the carbon in the ocean sediments and the action of volcanos, but these are very important if we are to have a good understanding of global warming.
Carbon exists in the atmosphere mainly in the form of carbon dioxide, but with small amounts of methane. Samples from the Greenland Ice Cores show that for at least the past hundred thousand years and until about 1800 carbon dioxide has made up about 0.03% of the Earth’s atmosphere. Since about 1800 it has been rising, almost certainly as the result of Man’s burning of fossil fuels. We know from other sources that much further in the past it was significantly higher or lower. The level of carbon dioxide in the atmosphere affects the way the Earth radiates heat into space and so regulates the temperature of the Earth. This is discussed much more fully on other Pages.
Plants remove carbon dioxide from the atmosphere by a process called photosynthesis. The word photosynthesis comes from the Greek words for making with light.
The plant obtains the water needed for photosynthesis from the soil through its roots. The glucose becomes the plant’s food; the oxygen is a waste product which is returned to the atmosphere.
This process needs energy which the plant obtains from sunlight. Plants therefore photosynthesise only by day.
A few other organisms, such as algae and lichens, also photosynthesise, but their role in the Carbon Cycle is the same as that of plants so they are included with plants on this Page.
They obtain the oxygen from, and return the carbon dioxide to, the atmosphere.
Plants respire by day and by night, but photosynthesise only by day. But during the day they photosynthesise far more than they respire, enough in fact to produce all the oxygen (and also all the food) needed not for their own respiration but also that of all other living things for all day and all night.
Some of the carbon dioxide a plant removes from the atmosphere by photosynthesis is returned to the atmosphere as carbon dioxide by the plant’s respiration, and some of it is built up by the plant into very complex organic molecules such as proteins. To make these the plant needs not only carbon, oxygen and hydrogen, which it gets from water and carbon dioxide, but also other elements such as nitrogen, phosphorus and potassium. These it obtains from the soil through its roots in the form of simple soluble inorganic molecules. More information about this is given in the Page on Plant Nutrition.
The plant needs energy to make these organic molecules, which it obtains from its own respiration. These molecules, and of course the carbon atoms in them, then become part of the plant.
Part or all of the plant may then be eaten by an animal. Some of the complex organic molecules in the plant may then become part of the animal’s body, some may be used by the animal to produce energy by respiration (in which case the carbon is returned to the atmosphere as carbon dioxide), and some may be egested by the animal in its faeces. The faeces in their turn may be eaten by dung beetles and maggots and other organisms. Some animals such as snails, shellfish and birds also use carbon (and of course calcium) to make calcium carbonate for their own shells or the shells of their eggs. It should be noted that the bones of vertebrates, including mammals and birds, contain calcium phosphate, not calcium carbonate.
The animal may be eaten by another animal, and so on.
When a land plant or animal (or any other land organism) dies any parts which have not been eaten will usually fall to the ground and go into the soil. Here the complex organic molecules may be broken down by decomposing organisms such as fungi and bacteria. As the dead organism decomposes some of the carbon in the complex organic molecules may be released back into the atmosphere as carbon dioxide, or sometimes as methane - this is eventually oxidized to produce carbon dioxide. Decomposition may also break down the complex organic molecules in a way which converts the nitrogen, phosphorus and potassium etc in them into the simple inorganic molecules which the plants can re-use.
The decomposing organisms do of course themselves have structures made of complex organic molecules and obtain their own energy by respiration. We think of mushrooms and toadstools as fungi, but they are only what is called the fruiting body (like the flower of a plant) - the main part of most fungi consists of threads of mycelium spreading through the soil (or tree, dead animal, loaf of bread etc) and sometimes covering several hectares: although bacteria and fungi are both decomposing organisms, bacteria are among the world’s smallest organisms and fungi are among its largest!
Animals such as vultures eat dead meat. Dead meat is called carrion, and carrion-eaters are called scavengers. But although scavengers, and also animals such as dung beetles and earthworms, are enormously important they are not decomposers: decomposers are very specifically the organisms which convert complex organic molecules into much simpler water-soluble inorganic molecules so that the nitrogen, phosphorus and potassium etc in them can be re-used by plants.
Often however dead animals or plants or other organisms may become buried in the soil in a way which prevents complete decomposition, so that the carbon in some of the complex organic molecules is not released as carbon dioxide or methane, instead it may be stored in the soil, and perhaps eventually in rocks deep underground, for thousands or millions of years in the form of much simpler organic molecules. This reduced carbon (that is, compounds of carbon not containing oxygen, so not in the form of carbon dioxide or carbonates) includes fossil fuels such as coal, petroleum and natural gas. However, only about 3% of the reduced carbon in the Earth’s crust is in the form of fossil fuels, the remainder is mixed in the soil and subsoil and underlying rocks. In the soil it is not itself a plant nutrient but it is a vital part of the soil because it helps the soil retain nutrients. If the soil becomes degraded by poor farming methods, particularly after deforestation, the carbon in this reduced carbon may become oxidised and returned to the atmosphere as carbon dioxide, so contributing to global warming.
The shell of an animal may be broken and crushed when the animal dies or is eaten, and many animals (including rats) eat shells, particularly egg shells or even whole eggs, to obtain the calcium they need - that is why pigeons’ nests attract rats and why you should not put egg shells onto compost heaps. The acid in the animal's stomach dissolves the calcium carbonate releasing the carbon as carbon dioxide. (Egg shells are not poisonous to Man but their sharp edges can cause damage to the stomach lining and most people do not eat them.) But the calcium carbonate in shells which go into the ground is not broken down by decomposing organisms, and the carbon in it may remain locked up in it for many, perhaps millions, of years. What happens to calcium carbonate in the Earth’s crust is discussed in the Page on Limestone.
Both calcium carbonate from animal shells and all forms of reduced carbon may be taken deep into the Earth’s mantle by tectonic movements at subduction zones, where one tectonic plate is sliding under another. The intense heat in the mantle breaks them down and the carbon in them is then released back into the atmosphere by volcanos as carbon dioxide. Volcanos are an integral and very important part of the carbon cycle although this is not always made clear. This is discussed in greater detail on another Page.
We call this planet Earth because we live on the dry land. But more than two thirds of the surface of the planet is covered with water. Seen from space it is a most beautiful blue colour: a visitor from another planet would probably call it not Earth but Ocean.
The carbon cycle in water follows the same pattern as that on land, except that the carbon dioxide produced by respiration and needed for photosynthesis is dissolved in the water. In the deep oceans the only plants (producers) are the phytoplankton, very tiny floating plants. These are eaten by the zooplankton, very tiny animals, which in turn are eaten by larger animals. The phytoplankton and zooplankton together make up the plankton layer. This is only a few metres thick because of the need for light for photosynthesis.
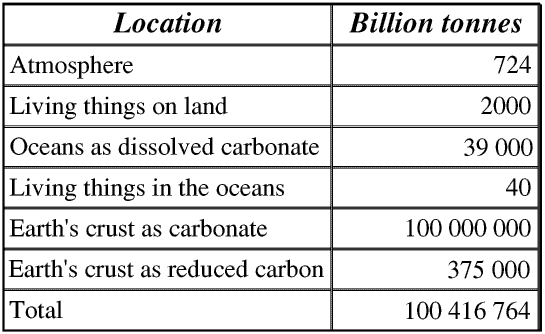
Here is a table showing the distribution of carbon in living things and the Earth’s atmosphere, oceans and crust.