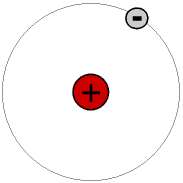
All atoms consist of a central nucleus with one or more electrons in one or more shells around it. The distance between the electrons and the nucleus is very large compared with the size of the nucleus and electrons: like the Solar System, an atom is mostly empty space!
The simplest atom is the commonest isotope of hydrogen, atomic symbol H (isotopes are explained later). This has a nucleus containing just one particle, a proton, and one electron in a shell round it. A proton has a positive electrical charge and an electron an identical negative electrical charge, so the atom as a whole has no electrical charge. The mass of a proton is about two thousand times that of an electron.

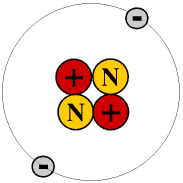
The next simplest atom is the commonest isotope of helium, atomic symbol He. This has two protons in the nucleus and two electrons in a shell round it. A proton has a positive charge, so two protons would repel each other and a nucleus containing just two protons would be unstable and eventually disintegrate. The nucleus of this helium atom therefore contains two neutrons as well as two protons. Neutrons have no electrical charge and almost exactly the same mass as protons. Their purpose is to provide a binding force which overcomes the electrostatic force between the protons and so keep the nucleus stable.
The next simplest atom is the commonest isotope of lithium, atomic symbol Li. This has a nucleus containing three protons and four neutrons, and three electrons in shells round it.
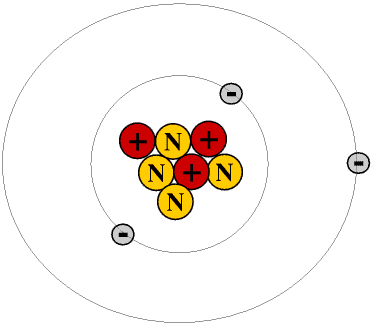
The electrons are arranged round the nucleus in shells. The way the electrons are arranged in these shells is very important indeed because it determines the chemical properties of the element but is not considered further here.
The particles (protons and neutrons) which make up the nucleus of an atom are called nucleons. The number of neutrons is given the symbol N and the total number of nucleons is called the nucleon number, with the symbol A. So A = N + Z. A is shown as a prefix superscript to the atomic symbol like this
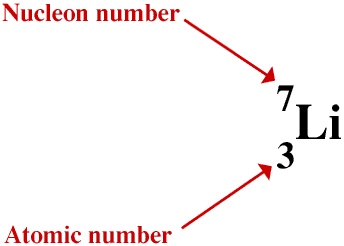
But we do not usually show both the atomic number and the nucleon number with the atomic symbol in this way unless there is a reason why we should, for example when writing symbolic equations for nuclear reactions, partly because it can be confusing and partly because it is not easy to include both at the same time using a computer word processor or HTML generator. But we must always include at least the nucleon number when writing about nuclides and isotopes - this is discussed below.
Protons and neutrons have about the same mass, and electrons about 0.0005 of this, so to a first approximation an atom of helium, with two protons and two neutrons and two electrons, is about four times heavier than an atom of hydrogen, with just one proton and one electron. So we often refer to the nucleon number as the mass number.
But a neutron and a proton do not have exactly the same mass, and an electron does have some mass, so the mass of an atom of ordinary helium is not exactly four times the mass of an atom of ordinary hydrogen. Although this difference is very tiny indeed it is also very important indeed: deep inside stars four ordinary hydrogen atoms (a total of four protons and four electrons) become one ordinary helium atom (consisting of two protons, two neutrons and two electrons). The nucleon number is conserved but the mass is not, and the very tiny loss in mass, which occurs when two protons and two electrons become two neutrons, releases a far from tiny amount of energy, according to Einstein’s famous equation E = m × c2. All the heat and light of the Sun comes from the energy released as it turns hydrogen into helium, losing mass as it does so. It is actually losing mass at about five million tonnes a second - about the rate at which you could empty the North Sea with a teaspoon! This is more fully discussed on the Page on Atomic weight and the mole
The neutrons in the nucleus of an atom are there to make it stable: if they were not there the positive charges on the protons would cause them to repel each other and the nucleus would eventually disintegrate. The nucleus of the commonest type of hydrogen atom, hydrogen-1 (note the hyphen), or 1H, does not need and does not have a neutron, but hydrogen also exists as hydrogen-2 (2H) atoms, with one proton and one neutron, and hydrogen-3, (3H) atoms, with one proton and two neutrons. Ordinary hydrogen is about 99.99% hydrogen-1.
A nuclide is an atom or nucleus with a certain atomic number and a certain nucleon number, for example hydrogen-2 is a nuclide. Atoms with the same number of protons but different numbers of neutrons are called isotopes, for example hydrogen-1, hydrogen-2 and hydrogen-3 are isotopes. There is often confusion between nuclides and isotopes, but you will not get confused if you remember that the isotopes of an element are members of a family, like brothers. You cannot say “An isotope is ...” any more you can say “A brother is ...” - you must say “Isotopes are ...” and “Brothers are ...”.
We must always show the nucleon number when discussing nuclides and isotopes. The neutrons are there to make the nucleus stable by counteracting the electrical repulsion between the protons, but only certain arrangements of protons and neutrons are stable. Nuclides with unstable nuclei are radioactive and may spontaneously disintegrate. Hydrogen-3 is radioactive. Unstable nuclides are called radionuclides, or sometimes radioisotopes.
Hydrogen-2 is commonly called heavy hydrogen, or deuterium, and given the symbol D. We also give hydrogen-3 the symbol T, for tritium. Heavy water (water containing hydrogen-2 rather than hydrogen-1, that is, D2O) was very important in the development of the atom bomb during the Second World War because it could be used to control the rate of the nuclear reactions in a nuclear reactor (originally called an atomic pile). Today other substances are used in nuclear reactors although heavy water is still used in many nuclear research programmes. This is further discussed on the Nuclear Fission Page.
Helium exists mainly as helium-4, with about one part in a million of helium-3. Neither are radioactive. There are also minute amounts of isotopes with higher nucleon numbers, such as helium-5 and helium-6. These are radioactive.
The nuclei of all carbon atoms (6C) contain 6 protons but carbon atoms may be carbon-12, with six protons and six neutrons, carbon-13 with 7 neutrons, or carbon-14 with 8 neutrons. Carbon-14 is radioactive; it is very important because it is the basis of carbon dating archaeological objects.
We compare the masses of different atoms by referring to their relative atomic mass. It should be noted that the atomic mass unit is actually defined as 1/12 of the mass of a carbon-12 atom rather than the mass of a hydrogen-1 atom, although if we take the relative atomic mass of an atom to be the same as the nucleon number, which for many purposes we usually do, this is of no practical significance.
If we take a bag containing a hundred 1p coins and weigh it, the mean (average) mass of all of the coins in the bag will be the actual mass of a 1p coin. If we now take out a 1p coin and replace it with a 2p coin, the mean mass of the coins in the bag will be very slightly more than that of a 1p coin.
All elements except fluorine occur naturally in a form which contains at least two isotopes. For example magnesium (12Mg) occurs naturally in a form containing 79% magnesium-24, 10% magnesium-25 and 11% magnesium-26 - none of them are radioactive. We can use these figures to calculate the mean mass of an atom of magnesium - this is the (mean) relative atomic mass, with the symbol Ar. For magnesium this is 24.305. The mean relative atomic mass of an element is also called its atomic weight, and this is a perfectly correct alternative to mean relative atomic mass. But atomic mass by itself is not acceptable. Most people except me and my students miss out the mean in mean relative atomic mass. This is further discussed on the Page on Atomic Weight and the Mole.
Although all elements except fluorine occur naturally in a form consisting of several isotopes, for many elements one isotope is more common than the others, and therefore the mean relative atomic mass of such an element will be close to the mass (nucleon) number of the most common isotope. So the values of Ar published in tables intended for use by students will often be rounded to this value.
One exception is chlorine (17Cl), which consists of about 75% chlorine-35 and 25% chlorine-37, so Ar for chlorine is about 35.5. Another exception is copper (29Cu) which consists of about 70% copper-63 and 30% copper-64 so its Ar is about 63.5.
Here are some common elements and their approximate mean relative atomic masses (atomic weights)
For a list of (most of) the chemical elements go to The Chemical Elements; this Page provides a link to a Periodic Table web site which gives Ar for every element to better than four decimal places.
For schoolwork and in exams you will usually be given the value of Ar you will be expected to use for the elements you need, you will not be expected to know them by heart.
Remember that correctly the relative atomic mass refers only to a particular nuclide, for example magnesium-24. The mean relative atomic mass (or atomic weight), Ar, refers to the mean relative atomic mass of all the atoms in an element, for example magnesium.
There is more about atomic weight on the Atomic Weight and the Mole Page.
© Barry Gray April 2016