Archaeologists, historians, antique dealers, librarians, museum curators and many other people often need to date things. There are many different ways of doing this. You can use carbon or other methods based upon radioactivity, and if that is your only interest you can go straight there. But I hope very much that either now or later you will look at the rest of this Web Page to realise that there are other methods of dating things.
“This Chest has been in my family for 450 years. It was made for the 3rd Duke in 1551 by William Smith of Shrewsbury - here is the original Bill of sale for four pounds three shillings and sixpence dated 14th March 1551. In 1673 the 6th Duke had his portrait painted standing in front of it - here is the portrait together with the artist’s signed and dated receipt. In 1788 the Hall Library was damaged by a slight fire - here is the list of books and furniture that were rescued from the library. In 1852 the Chest was mentioned in my great grandfather's Will - here is the Will. In 1892 the Prince of Wales visited the Hall - here is a photograph of him admiring the Chest. During the Second World War the Chest was moved to a safe place in South Wales - here is the receipt from the removal company. In June 1946 it was brought back to the Hall - here is the cutting from the local newspaper covering the event.”
Mind you, few objects would have a provenance that good!
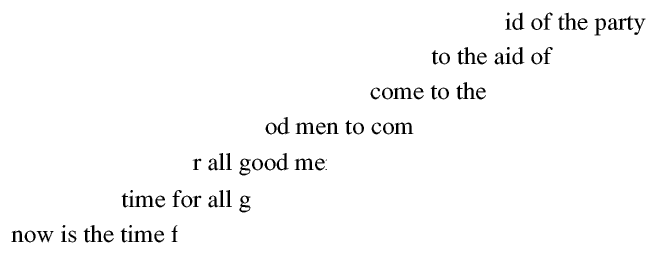
Over the years scientists have built up a data bank for almost every species of tree and almost every country for at least a thousand years and for some tree species more than ten thousand years: from this data bank they can date almost any large piece of wood, from a ship or a building, for the whole of the historical period - if the core from the sample contains the bark we know when it was cut down to an accuracy of one year! The longer the core the greater the number of rings and so the more likely we are to find a correct match.
We can also study the weather pattern in Antarctica in the same way by drilling into the ice to obtain a core, except that here the cores are thousands of metres long and cover hundreds of thousands of years!
Pottery is enormously important to archaeologists. When Man first started to collect and store food he needed ways of carrying it and protecting it from rats and other wild animals. There was no Tupperware or stainless steel; instead he used bags made from animal skins, baskets woven from grasses and twigs, and pots made from fired clay.
If you have ever watched an antiques programme on television you may have seen The Expert pick up a piece of china and just by looking at it say when it was made and where it was made. And this has always been the case: every period in history and every place on Earth has always had its own distinct type of pottery. So a pot, or even a piece of broken pot (a potsherd or just sherd) gives an archaeologist a lot of information. Often a piece of broken pot gives even more information: writing materials such as papyrus and parchment were very expensive, far too expensive to give to children learning to write or for things like shopping lists. So children practiced writing and adults made shopping lists on pieces of broken pot, and pieces of broken pot last far longer than pieces of parchment or papyrus: today we can read stories copied down by Ancient Egyptian children onto broken pots more than a thousand years before the time of King Tutankhamen. Bits of broken pot with writing on are called ostraca.
Pottery is discussed further on the Clay In The Ancient World Page.Early Man made clothes mainly out of fur and leather and other animal products such as bone. By identifying the leather we can tell what sorts of animals he was hunting. The marks left by his tools on the leather may also tell us how he had killed the animal and cut up the carcass and made the leather into clothes.
Later he began to make clothes out of natural fibres such as cotton, wool, silk and linen. Here the fibres must first be spun to make them stick together to turn them into long threads, and then the threads must be woven together on a loom to make them into cloth. You can probably see the criss-cross pattern of threads in the clothes you are wearing now (except knitted clothes) or your handkerchief. Then the cloth can be cut into pieces and the pieces joined together to make clothes. We can learn a lot about the way the fibres were spun and the cloth was woven by looking at a small piece under a microscope, and this, and the way the pieces were joined together, enables us to date even a tiny piece of cloth or clothing.
Buckles were often made of metal, and gold belt buckles are often found in the graves of kings and other rich people, but most fastenings were made of bone, wood or ivory, and these give us lots of information too.
Cloth is very important to archaeologists for another reason: smoke and dust, pollen and many other very fine particles get caught in the fibres, and we can gets lots of information from examining these particles. Pollen is particularly important. Flowering plants, including trees, grasses and most plants grown for food, produce huge amounts of pollen. This is a very fine powder and blows everywhere. But if you look at pollen grains under a powerful microscope you will see that they have the most wonderful shapes, and every species of plant has its own distinctive shape. An expert can look at one grain of pollen stuck in the fibres of a piece of cloth and tell you what sort of plant it came from, even after more than five thousand years. If for example we find grass pollen the people were probably keeping sheep, wheat pollen would tell us they were farmers. Some plants grow only in certain places and this helps us to identify where the cloth was made, or at any rate where it has been.
If a piece of cloth has been in a stone building such as a church it may trap particles of dust that have flaked off the stones. Limestone has often used for building. It is mainly calcium carbonate but it also contains minute amounts of other substances. These substances colour the limestone: limestone from different places is different colours because it contains different substances. By analysing the limestone dust found on the cloth we can tell where the limestone came from.
If we just leave a building or an object, over a period of years it will be buried under other material: the longer it is left the deeper it will be buried. If you watch archaeologists excavating a site you will see them digging trenches across it. This allows them to find how deep each object is buried. If they find one object next to another object which can be dated this helps them to date the first object.
Today’s archaeologists are looking mainly for information; treasure seekers are looking for (surprise!) treasure. The tragedy today is that many treasure seekers with metal detectors are looking for gold and silver and other metal objects and do not always understand the importance of recording, particularly by photographing, other objects in their positions. They dig up a handful of coins without bothering about bits of broken pot or anything else, and by doing so destroy almost all of the archaeological information the site contains.Many objects can be dated using methods involving radioactivity. There are four main methods: carbon, uranium-lead, rubidium-strontium, and potassium-argon. Carbon dating can be used to determine the age of organic remains less than about 50 000 years old and is widely used by archaeologists; the other three are used mainly by geologists to find the age of igneous rocks. You do not need to know much about radioactivity except the idea of radioisotopes and half-life to understand what follows, but if you want to find out more about radioactivity you can go to my Web Page on radioactivity: to link to it please click here ![]()
Please be warned that if you search the internet for information on dating objects using methods based upon radioactivity you will find that some of the sites you will find are run by American Christian fundamentalist groups. These people believe that, according to a very literal interpretation of the first chapter of the first book of the Bible, the Earth was created by God in six days less than five thousand years ago. These web pages are therefore concerned almost entirely with arguing that the Earth is only about four thousand six hundred years old, not four thousand six hundred million years old, as radioactivity in rocks would indicate. Their beliefs of course mean that the dinosaurs never really existed!
We can use carbon dating to estimate the age of organic material less than about 50 000 years old: after ten half-lives the amount of carbon-14 remaining has fallen to less than a thousandth of its initial value and is too tiny to measure. Things which can be carbon dated include charcoal (very important because it is often left by fires used for cooking), wood from buildings and shelters, boats, furniture and wooden tools, arrows, leather and animal skins, clothes and textiles such as wool, silk, cotton and linen, animal and human hair, bone, egg shells, mummy wrappings, straw used for packing, parchment and papyrus, etc.
We can only use carbon dating for organic materials less than 50 000 years old. For example if we find a bead necklace we can only date the leather cord not the beads; coal and oil and things made from them contain carbon but this is much too old to date.
Carbon dating was first used in 1949. Initially it was based upon the assumption that the level of carbon-14 in the atmosphere had been the same in the past as it is today, but we now know that this is not the case. We can however apply a correction factor based upon dendrochronology - this takes us back to about 10 000 years ago. Beyond 10 000 years the results of carbon testing cannot at present be corrected for variations in the levels of carbon-14 in the atmosphere.
Carbon testing is a destructive process, that is, the sample being tested is completely destroyed. So you do not test an oak chest, you only test a very small piece of it! The first stage therefore is to select a sample. The methods used in carbon dating have changed greatly over the past forty years. In the early days several grams were required, but by the 1970s only a few milligrams were needed.
The next stage is to decontaminate it, that is, remove every trace of all other organic material from it. A piece of cloth two thousand years old will probably be covered with dirt and grime from every century since then, sweat from hands that have touched it, even soap that may have been used to wash it, plant pollen, dead dust mites (and their faeces!), a whole range of organic material which is more recent and which if not removed will seriously affect the result.
The sample is then heated in pure oxygen. This converts the carbon in it into carbon dioxide. This is then separated from all other substances.
The final stage is measuring the ratio of carbon-14 to carbon-12 in the sample. This will allow us to calculate its age. Originally, at a time when several grams of the sample were required, the carbon dioxide gas produced by the last process was reduced to carbon and its radioactivity measured. Since then other methods needing only a few milligrams of material have been developed. There is a very technical explanation of these different processes on the Radio Carbon Dating Web Site - to link to it please click here ![]()
Finally, it may be helpful to stress the enormity of the problem we are faced with. We are trying to measure the change in the ratio of carbon-14 to carbon-12 atoms over a number of years in a piece of material smaller than a postage stamp, when even in a brand new piece of the material the carbon-12 atoms outnumber the carbon-14 atoms by a factor of more than a trillion!
Igneous rocks are formed when molten material (“magma”) cools. Depending upon the conditions as it cools, the magma may be pulled by gravity into large horizontal sheets or surface tension may pull it into smaller approximately spherical geodes.
What happens as the magma cools is easiest to explain if we think about geodes.
The magma is a very complicated mixture of many different minerals, all with different melting points, and dissolved gases. As it cools the mineral with the highest melting point starts to form crystals first. These form a solid layer round the outside of the geode. The size of the crystals depends upon the rate of cooling, so these first crystals will be quite small. Solid rock is a very poor conductor of heat, so as the solid crust gets thicker the rate of cooling of the inside will get slower and bigger crystals will be formed. Each mineral will start to crystalize when the inside of the geode reaches the melting point for that mineral. When all the minerals have solidified there is a space inside the geode which is filled with the gases which were dissolved in the magma when the crust first formed.Here is a photo of a geode: you can see the layers of different minerals quite easily, and also see how the size of the crystals changes.

Not all spherical rocks are geodes. If we have a china teapot the parts most likely to get damaged are the sticky-out bits, that is, the spout and handle. Similarly if we have a large pile of stones of different shapes and tumble them together the parts that are most likely to get knocked off are the sticky-out bits, and after a time there won't be any sticky-out bits left, that is, they will all be smooth and round. Hence the pebbles on the seashore.
Rubidium is the twenty second most common element in the Earth's crust. It exists as two isotopes, 72% rubidium-85 and 28% rubidium-87. Rubidium-85 is not radioactive but rubidium-87 is, it is a beta emitter with a half-life of 47.5 × 109 years, and decays to form strontium-87. This is not radioactive. The rubidium-87 will be decaying to form strontium-87 even in the magma. However minerals containing rubidium have melting points different from those containing strontium so will crystalize at different temperatures. This means that at the time the rock is formed the rubidium crystals will not contain any strontium. Once the rock has been formed the decay products (in this case strontium-87) are trapped within the crystal. So the amount of strontium-87 within the crystals enables us to calculate the age of the rock.
Similarly, radioactive potassium-40 decays to form non-radioactive argon-40 so we can date rocks by measuring the amount of argon-40 in them.
Similarly uranium-238 decays to form lead-206, uranium-235 decays to form lead-207, and thorium-232 decays to form lead-208, so these can also be used to date rocks. However in these cases many other radioactive substances are produced on the way so the calculations are much more difficult - there is more about this on the Radioactivity Page.
Igneous rocks were formed when the Earth's crust first started to develop, so some igneous rocks are very old indeed. But they are also formed by volcanoes and at tectonic plates boundaries, so some igneous rocks are very young! Because of the way they are formed you do not find fossils or organic material in igneous rocks.